SBA-15
Hydrothermal Method
Product Detail
Santa Barbara Amorphous-15 (SBA-15) is a highly stable mesoporous silica sieve developed by researchers at the University of California at Santa Barbara. It gets it high hydrothermal and mechanical stability from a framework of uniform hexagonal pores that feature a narrow pore-size distribution and a tunable pore diameter of 5nm to 15nm, but, most significantly, from its relatively thick walls, which range between 3.1nm and 6.4nm. SBA mesoporous silica 15 has a high internal surface area, which lends itself to various applications, including environmental adsorption and separation, advanced optics, and catalysts.
Mesoporous silica SBA-15 is synthesized in acidic conditions using triblock copolymer Pluronic 123 (EO20PO70EO20) as a pattern and tetraethoxysilane (TEOS) for the source of silica. Once synthesis is complete, the organic template is removed by a variety of methods, including calcination; reflux extraction; H2O2 treatments; microwave digestion; and/or washing with pure solvents, ethanol, acetone, or water. ACS Materials’ SBA-15 was calcined in air at 823 Kelvin for 6 hours, resulting in a fine, white powder.
ACS Materials provides the finest nanomaterials to commercial and academic research labs around the world. Our products are on the leading edge of technology and are contributing to advancements in the fields of biology, chemistry, physics, and engineering. Our goal is to provide the highest-quality products at the lowest possible prices and to provide exceptional customer service before and after each purchase. Contact us today to see how we can help your research reach new heights.
CAS No.: 7631-86-9
1. Preparation Method
Hydrothermal Method
2. Characterizations
Key features of our SBA-15 products are:
| Appearance: | White Powder |
| Particle Size (μm) | 1~4 |
| Pore Diameter (nm) | 6~11 |
| Pore Volume (cm3/g): | 1.46 |
| BET surface area (m2/g) | ≥550 |
| Phase | p6mm |
| Comparative Crystallinity (%) | ≥90 |
| BJH Desorption Cumulative Volume of Pores (cm3/g) |
0.6793 |
| Bulk Density (g/cm3) |
0.067 |
| Tap Density (g/cm3) | 0.145 |
This product is calcined and ready to use directly.

Typical SEM Image of ACS Material Mesoporous Silica Molecular Sieve SBA-15 (1)

Typical SEM Image of ACS Material Mesoporous Silica Molecular Sieve SBA-15 (2)
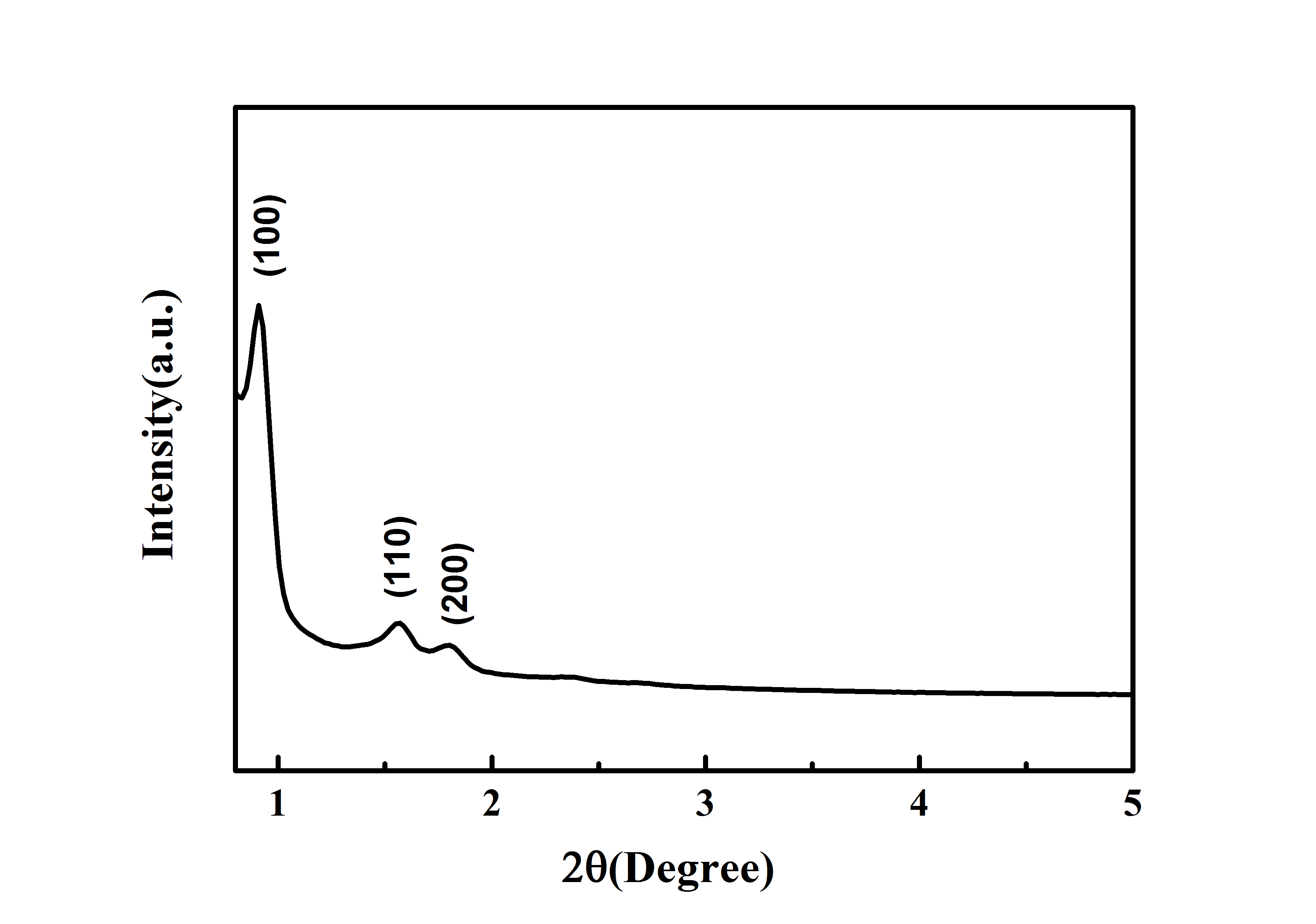
Typical XRD Analysis of ACS Material Mesoporous Silica Molecular Sieve SBA-15
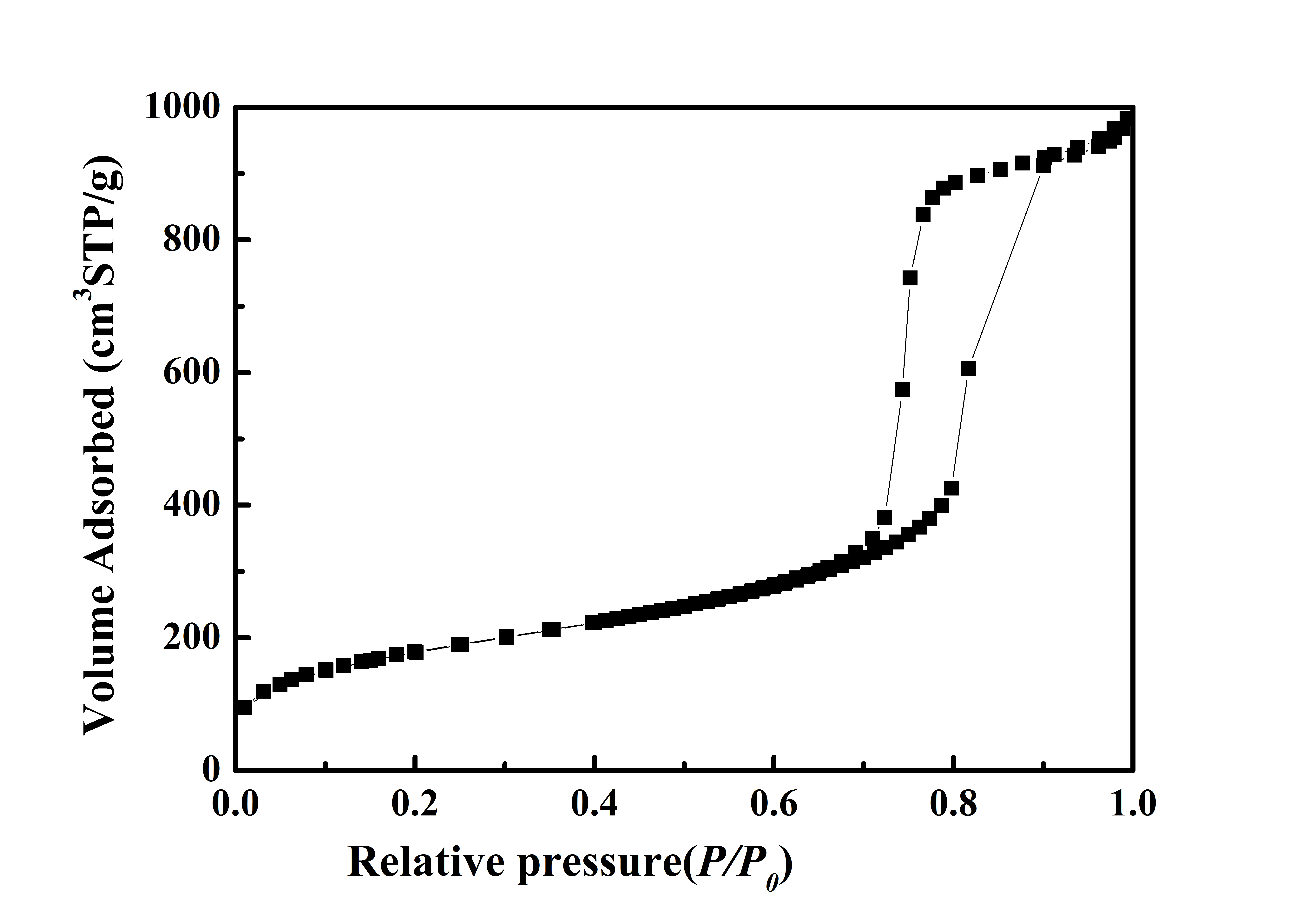
Typical BET Analysis of ACS Material Mesoporous Silica Molecular Sieve SBA-15
3. Application Fields
1) Catalyst carrier
2) As a templating agent
3) Nanomaterials
Disclaimer: ACS Material LLC believes that the information on our website is accurate and represents the best and most current information available to us. ACS Material makes no representations or warranties either express or implied, regarding the suitability of the material for any purpose or the accuracy of the information listed here. Accordingly, ACS Material will not be responsible for damages resulting from use of or reliance upon this information.
FAQs
1. What is the final calcination temperature used during synthesis?
The material is calcined in air at 823 Kelvin for 6 hrs to decompose the triblock co-polymer and yielding a white powder, SBA-15. This is the typical method for preparing SBA-15.
2. What is the collapsing temperature of SBA-15?
About 750 degrees Celsius.
3. What are the impurity elements in SBA-15?
The impurity is Na2O, whose content is less than 0.1%.
4. Which What method was used to measure pore volume?
We use a BET specific surface area test method. The instrument itself has a specific method for determining the pore volume.
5. Which BET method is used to calculate 1) Pore volume 2) Desorption cumulative volume of BJH pores?
We used BET test of liquid nitrogen adsorption. These two data are shown in the test results.
Research Citations of ACS Material Products
- Hong, Junghyun, and Francisco Zaera. “Interference of the Surface of the Solid on the Performance of Tethered Molecular Catalysts.” Journal of the American Chemical Society, vol. 134, no. 31, 2012, pp. 13056–13065., doi:10.1021/ja304181q.
- Wang, Huali, et al. “Support effects on hydrotreating of soybean oil over NiMo carbide catalyst.” Fuel, vol. 111, 2013, pp. 81–87., doi:10.1016/j.fuel.2013.04.066.
- Kuo, Yun-Hsuan, et al. “Concurrent Observation of Bulk and Protein Hydration Water by Spin-Label ESR under Nanoconfinement.” Langmuir, vol. 29, no. 45, 2013, pp. 13865–13872., doi:10.1021/la403002t.
- Goel, Jyoti, and Suddhasatwa Basu. “Effect of support materials on the performance of direct ethanol fuel cell anode catalyst.” International Journal of Hydrogen Energy, vol. 39, no. 28, 23 Sept. 2014, pp. 15956–15966., doi:10.1016/j.ijhydene.2014.01.203.
- Piumetti, Marco, et al. “Nanostructured ceria-Based catalysts for soot combustion: Investigations on the surface sensitivity.” Applied Catalysis B: Environmental, vol. 165, 2015, pp. 742–751., doi:10.1016/j.apcatb.2014.10.062.
- Lewandowski, Dawid, et al. “Fluorescence properties of riboflavin-Functionalized mesoporous silica SBA-15 and riboflavin solutions in presence of different metal and organic cations.” Journal of Physics and Chemistry of Solids, vol. 85, 2015, pp. 56–61., doi:10.1016/j.jpcs.2015.04.007.
- Skorupska, Ewa, et al. “NMR Study of BA/FBA Cocrystal Confined Within Mesoporous Silica Nanoparticles Employing Thermal Solid Phase Transformation.” The Journal of Physical Chemistry C, vol. 119, no. 16, Aug. 2015, pp. 8652–8661., doi:10.1021/jp5123008.
- Zandavi, Seyed Hadi, and C. A. Ward. “Nucleation and growth of condensate in nanoporous materials.” Physical Chemistry, vol. 17, no. 15, 2015, pp. 9828–9834., doi:10.1039/c5cp00471c.
- Lewandowski, Dawid, et al. “Immobilization of Zidovudine Derivatives on the SBA-15 Mesoporous Silica and Evaluation of Their Cytotoxic Activity.” Plos One, vol. 10, no. 5, May 2015, doi:10.1371/journal.pone.0126251.
- Lewandowski, Dawid, et al. “SBA-15 Mesoporous Silica Modified with Gallic Acid and Evaluation of Its Cytotoxic Activity.” Plos One, vol. 10, no. 7, July 2015, doi:10.1371/journal.pone.0132541.
- Bruzzoniti, M. C., et al. “Adsorption of bentazone herbicide onto mesoporous silica: application to environmental water purification.” Environmental Science and Pollution Research, vol. 23, no. 6, 2015, pp. 5399–5409., doi:10.1007/s11356-015-5755-1.
- Jabbari-Hichri, Amira, et al. “Effect of aluminum sulfate addition on the thermal storage performance of mesoporous SBA-15 and MCM-41 materials.” Solar Energy Materials and Solar Cells, vol. 149, 2016, pp. 232–241., doi:10.1016/j.solmat.2016.01.033.
- Gignone, Andrea. “Ordered Mesoporous Silica for Drug Delivery in Topical Applications.” Tesi di dottorato, 2016, p. 136., doi:10.6092/polito/porto/2652565.
- Melnichenko, Yuri B. “Structural Characterization of Porous Materials Using SAS.” Small-Angle Scattering from Confined and Interfacial Fluids, 2016, pp. 139–171., doi:10.1007/978-3-319-01104-2_7.
- Luo, Sheng, et al. “Confinement-Induced Supercriticality and Phase Equilibria of Hydrocarbons in Nanopores.” Langmuir, vol. 32, no. 44, 2016, pp. 11506–11513., doi:10.1021/acs.langmuir.6b03177.
- Welch, Ken, et al. “Investigation of the Antibacterial Effect of Mesoporous Magnesium Carbonate.” ACS Omega, vol. 1, no. 5, 2016, pp. 907–914., doi:10.1021/acsomega.6b00124.
- Krzyżak, A.t., and I. Habina. “Low field 1 H NMR characterization of mesoporous silica MCM-41 and SBA-15 filled with different amount of water.” Microporous and Mesoporous Materials, vol. 231, 2016, pp. 230–239., doi:10.1016/j.micromeso.2016.05.032.
- Yildiz, M., et al. “Support material variation for the MnxOy-Na2WO4/SiO2 catalyst.” Catalysis Today, vol. 228, 2014, pp. 5–14., doi:10.1016/j.cattod.2013.12.024.
- Skorupska, Ewa, et al. “Thermal Solvent-Free Method of Loading of Pharmaceutical Cocrystals into the Pores of Silica Particles: A Case of Naproxen/Picolinamide Cocrystal.” The Journal of Physical Chemistry C, vol. 120, no. 24, 2016, pp. 13169–13180., doi:10.1021/acs.jpcc.6b05302.
- Ferrer, Diana Iruretagoyena. “Layered Double Hydroxides Supported on Graphene Oxide for CO2 Adsorption.” Springer Theses (Recognizing Outstanding Ph.D. Research)., 2016, doi:10.1007/978-3-319-41276-4_5.
- Stanzione, M., et al. “Peculiarities of vanillin release from amino-Functionalized mesoporous silica embedded into biodegradable composites.” European Polymer Journal, vol. 89, 2017, pp. 88–100., doi:10.1016/j.eurpolymj.2017.01.040.
- Fiorilli, Sonia, et al. “Iron oxide inside SBA-15 modified with amino groups as reusable adsorbent for highly efficient removal of glyphosate from water.” Applied Surface Science, vol. 411, 2017, pp. 457–465., doi:10.1016/j.apsusc.2017.03.206.
- Dumitriu, Georgiana-Diana, et al. “Study of the potential use of mesoporous nanomaterials as fining agent to prevent protein haze in white wines and its impact in major volatile aroma compounds and polyols.” Food Chemistry, vol. 240, 2018, pp. 751–758., doi:10.1016/j.foodchem.2017.07.163.
- Yan, Y., et al. “Synthesis, stability and Li-Ion mobility of nanoconfined Li2B12H12.” Dalton Transactions, vol. 46, no. 37, 2017, pp. 12434–12437., doi:10.1039/c7dt02946b.
- Reiser, Sarah, and Michael Turk. “Influence of the Chemical Nature of Carrier Materials on the Dissolution Behaviour of Ibuprofen.” May 2016.
- Drobná, Helena, et al. “Analysis of Ni species formed on zeolites, mesoporous silica and alumina supports and their catalytic behavior in the dry reforming of methane.” Reaction Kinetics, Mechanisms and Catalysis, vol. 121, no. 1, Sept. 2017, pp. 255–274., doi:10.1007/s11144-017-1149-3.
- Diep, Brian. “Knowledge Bank.” Analyzing Catalyst Deactivation with a Packed Bed Reactor, The Ohio State University, 1 Dec. 2017, kb.osu.edu/dspace/handle/1811/81595.
- Madani, S. Hadi, et al. “Particle and cluster analyses of silica powders via small angle neutron scattering.” Powder Technology, vol. 327, 2018, pp. 96–108., doi:10.1016/j.powtec.2017.12.061.
- Bruzzoniti, Maria C., et al. “Polymer-Derived ceramic aerogels as sorbent materials for the removal of organic dyes from aqueous solutions.” Journal of the American Ceramic Society, vol. 101, no. 2, 2017, pp. 821–830., doi:10.1111/jace.15241.
- Zandavi, Seyed Hadi. “Vapours Adsorption on Non-Porous or Porous Solids: Zeta Adsorption Isotherm Approach.” TSpace, 1 Nov. 2015, tspace.library.utoronto.ca/handle/1807/71411.
- Dumitriu, Georgiana-Diana, Nieves López de Lerma, Valeriu V. Cotea, and Rafael A. Peinado. "Application of Mesoporous Materials as Fining Agents for Pedro Ximénez Wines." (2018).
- Weigler, Max, Martin Brodrecht, Gerd Buntkowsky, and Michael Vogel. "Reorientation of Deeply Cooled Water in Mesoporous Silica: NMR Studies of the Pore-Size Dependence." The Journal of Physical Chemistry B (2019).
- Afzal, Mohammad Suhail, Faiza Zanin, Muhammad Usman Ghori, Marta Granollers, and Enes Šupuk. "The effect of mesoporous silica impregnation on tribo-electrification characteristics of flurbiprofen." International journal of pharmaceutics 544, no. 1 (2018): 55-61.
- De la Torre Paredes, Cristina. "Nanotechnology and supramolecular chemistry in controlled release and molecular recognition proceses for biomedical applications." PhD diss., 2018.
- Iqbal, Muhammad Faisal, Satoshi Tominaka, Wenqin Peng, Toshiaki Takei, Nao Tsunoji, Tsuneji Sano, and Yusuke Ide. "Iron Aquo Complex as an Efficient and Selective Homogeneous Photocatalyst for Organic Synthetic Reactions." ChemCatChem 10, no. 20 (2018): 4509-4513.
- Bruzzoniti, Maria C., Marta Appendini, Luca Rivoira, Barbara Onida, Massimo Del Bubba, Prasanta Jana, and Gian Domenico Sorarù. "Polymer‐derived ceramic aerogels as sorbent materials for the removal of organic dyes from aqueous solutions." Journal of the American Ceramic Society 101, no. 2 (2018): 821-830.
