MCM-41
Hydrothermal Method
Product Detail
CAS No.: 7631-86-9
Please contact us for quote if your order quantity is larger than 1kg.
Mobil Composition of Matter No. 41 (MCM-41) is a mesoporous material developed by the Mobil Oil Corporation. MCM-41 is part of a family of silicate and alumosilicate solids that are well-suited for use as catalysts and catalytic supports.
Structure & Synthesis
MCM-41 has a hierarchical structure that has, as its base, an ordered arrangement of cylindrical mesopores that range in diameter from 2nm to 6.5nm. These independently adjustable mesopores form a unique, one-dimensional pore system that has sharp, well-defined pore distribution and large surface and pore volume.
During the synthesis of MCM-41, surfactants (typically cetyltrimethylammonium bromide (CTAB)) are added to the synthesis solution. The surfactant initially forms rod-shaped micelles that eventually align together in hexagonal arrays. Silica species are added to cover the rods, and then calcination condenses the silanol groups, which bridges the silicon atoms with oxygen atoms. Ultimately, this organic template oxidizes and disappears, leaving behind fully-formed MCM-41.
Properties & Uses
Because there is no aluminum in the lattice framework, MCM-41 has no Bronsted acid centers and has an acidity analogous to amorphous alumosilicates. The low level of cross-linking between silicate units and its thin walls mean MCM-41 is not hydrothermally stable.
MCM-41 is widely used in catalytic cracking, separations, dye removal, and as an adsorbent.
1. Preparation Method
Hydrothermal Method
2. Characterizations
|
Type: |
A |
B |
|
Grade: |
Reagent Grade |
Industrial Grade |
|
Appearance: |
White Powder |
White Powder |
|
Particle Size: |
100-1000 nm |
200-1000 nm |
|
Average Pore Diameter: |
3.4 nm |
3.5-4.0 nm |
|
BET surface area (m2/g): |
>850 |
>800 |
|
Pore Volume(cm3/g): |
≥0.75 |
≥0.75 |
|
Na2O: |
≤0.5% |
≤0.5% |
NOTE:
| Product: MCM-41 | A-20g | B-50g | B-100g |
| Old SKU#: |
MCM41-B-100g |
||
| New SKU#: | MSM41A01 | MSM41B15 | MSM41B21 |
This product is calcined and ready to use directly.
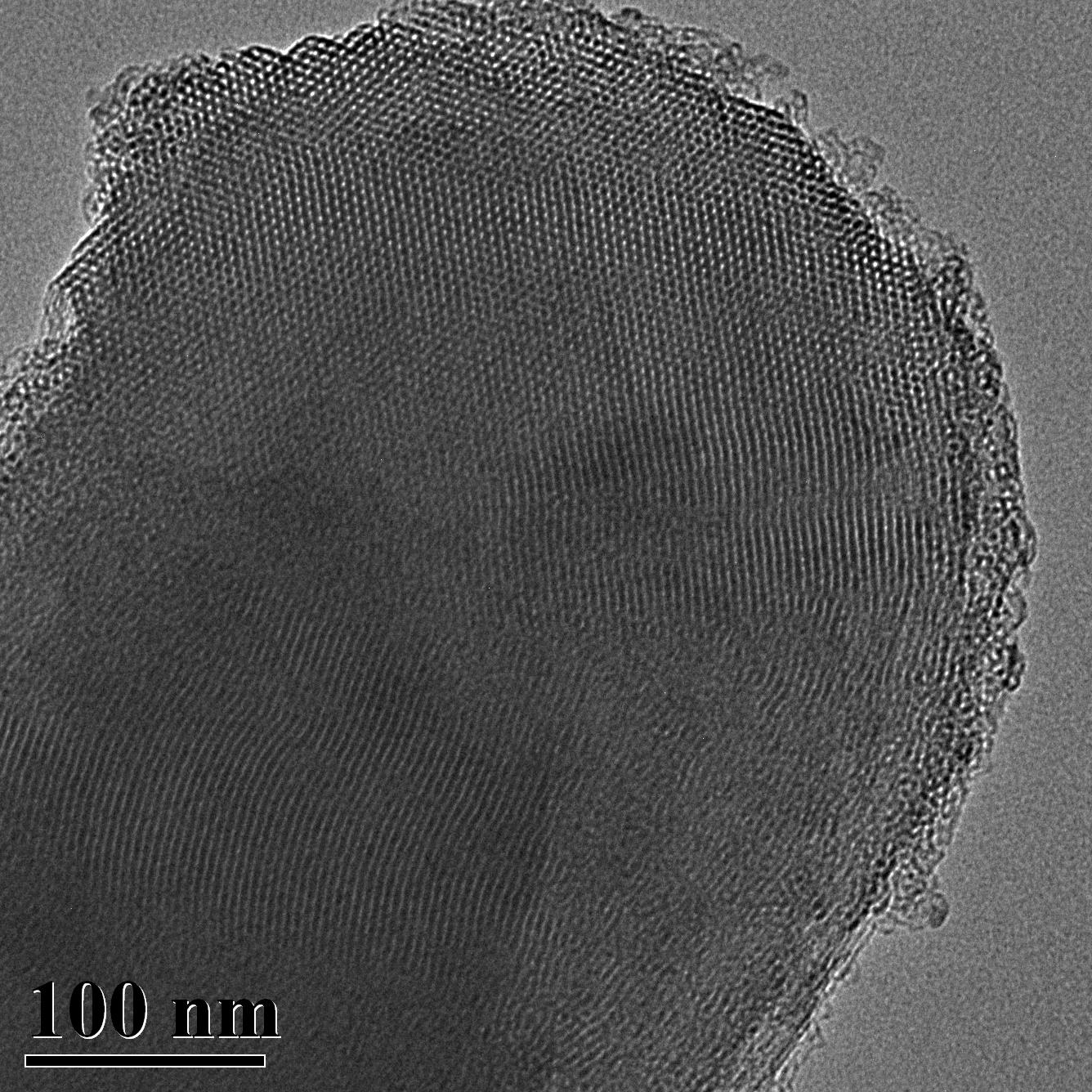
TEM Image (1) of ACS Material MCM-41 (Type A)
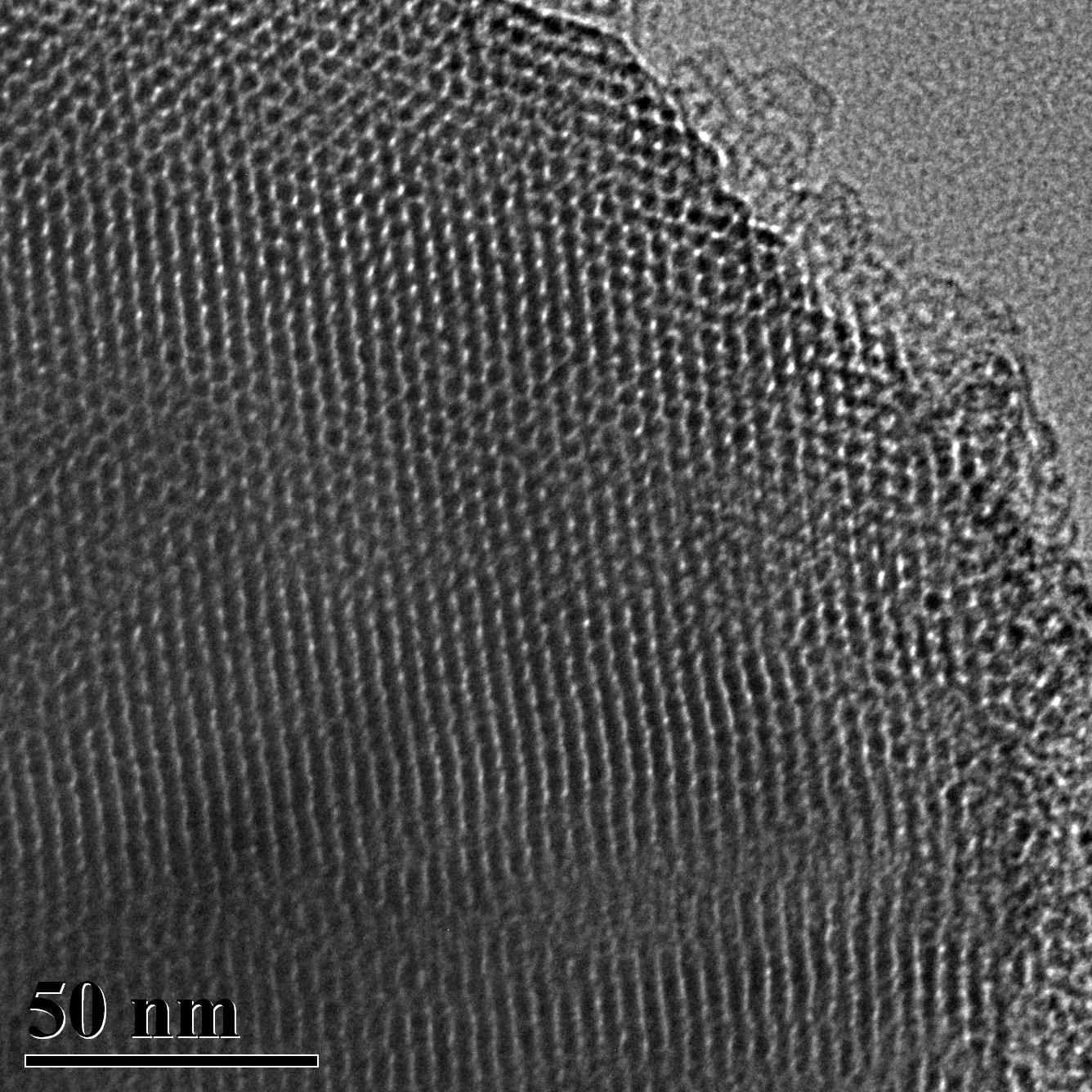
TEM Image (2) of ACS Material MCM-41 (Type A)
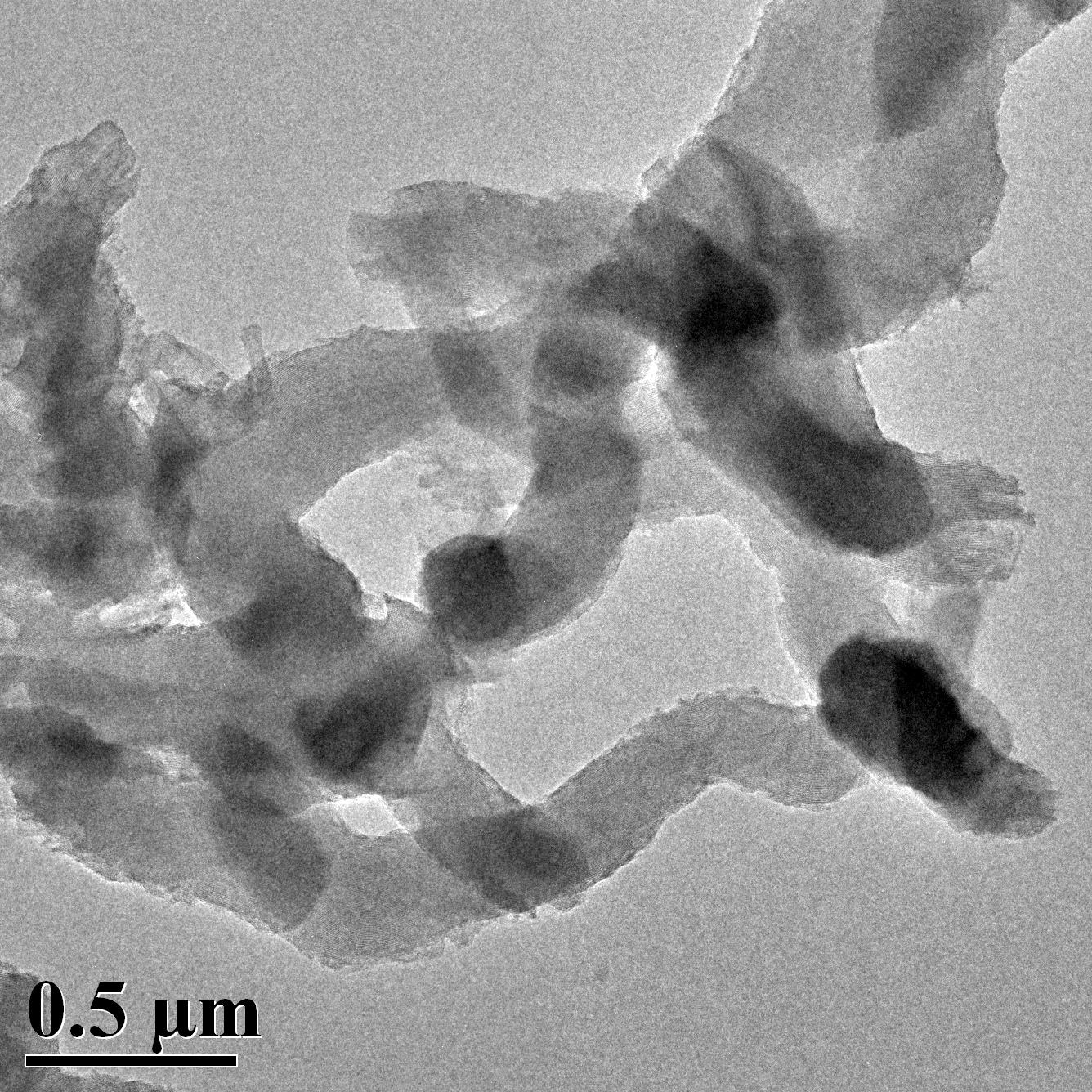
TEM Image (3) of ACS Material MCM-41 (Type A)
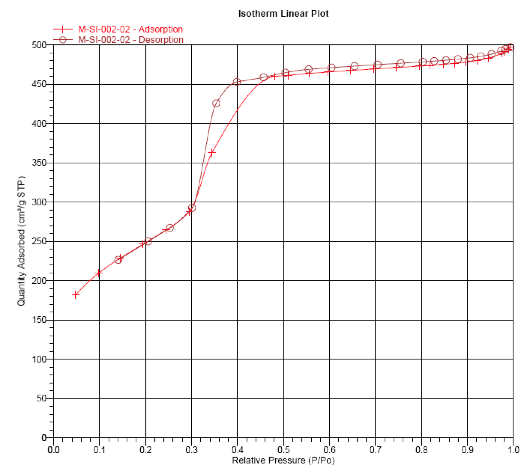
BET Analysis of ACS Material MCM-41 (Type A)
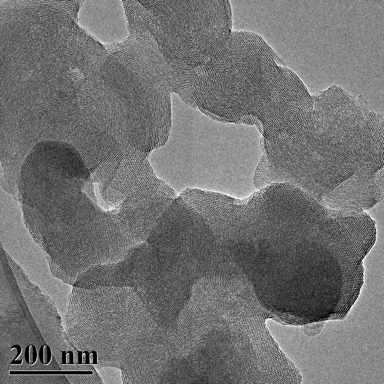
TEM Image (1) of ACS Material MCM-41 (Type B)
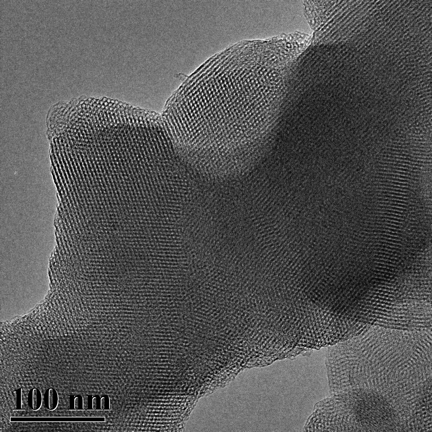
TEM Image (2) of ACS Material MCM-41 (Type B)
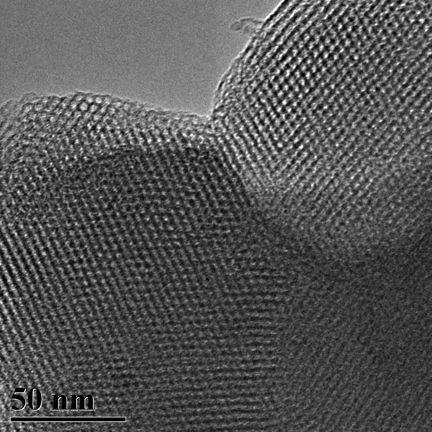
TEM Image (3) of ACS Material MCM-41 (Type B)
3. Application Fields
1) Catalyst
2) Dyes removal
3) Adsorbent
Disclaimer: ACS Material LLC believes that the information on our website is accurate and represents the best and most current information available to us. ACS Material makes no representations or warranties either express or implied, regarding the suitability of the material for any purpose or the accuracy of the information listed here. Accordingly, ACS Material will not be responsible for damages resulting from use of or reliance upon this information.
Research Citations of ACS Material Products
- Siahaan, Evi Amelia, et al. “Controlled release of allyl isothiocyanate from brown algae Laminaria japonica and mesoporous silica MCM-41 for inhibiting food-Borne bacteria.” Food Science and Biotechnology, vol. 22, no. S1, 2013, pp. 19–24., doi:10.1007/s10068-013-0043-7.
- López-Aranguren, Pedro, et al. “A new method using compressed CO2 for the in situ functionalization of mesoporous silica with hyperbranched polymers.” Chemical Communications, vol. 49, no. 100, 2013, p. 11776., doi:10.1039/c3cc47931e.
- López-Aranguren, P., et al. “Regenerable solid CO2 sorbents prepared by supercritical grafting of aminoalkoxysilane into low-Cost mesoporous silica.” The Journal of Supercritical Fluids, vol. 85, 2014, pp. 68–80., doi:10.1016/j.supflu.2013.10.020.
- López-Aranguren, Pedro, et al. “Understanding the Performance of New Amine-Functionalized Mesoporous Silica Materials for CO2 Adsorption.” Industrial & Engineering Chemistry Research, vol. 53, no. 40, 2014, pp. 15611–15619., doi:10.1021/ie502945r.
- Zandavi, Seyed Hadi, and C. A. Ward. “Nucleation and growth of condensate in nanoporous materials.” Physical Chemistry, vol. 17, no. 15, 2015, pp. 9828–9834., doi:10.1039/c5cp00471c.
- Saliba, Sarmenio, et al. “Combined influence of pore size distribution and surface hydrophilicity on the water adsorption characteristics of micro- and mesoporous silica.” Microporous and Mesoporous Materials, vol. 226, 2016, pp. 221–228., doi:10.1016/j.micromeso.2015.12.029.
- Melnichenko, Yuri B. “Structural Characterization of Porous Materials Using SAS.” Small-Angle Scattering from Confined and Interfacial Fluids, 2016, pp. 139–171., doi:10.1007/978-3-319-01104-2_7.
- Bruzzoniti, M. C., et al. “Adsorption of bentazone herbicide onto mesoporous silica: application to environmental water purification.” Environmental Science and Pollution Research, vol. 23, no. 6, 2015, pp. 5399–5409., doi:10.1007/s11356-015-5755-1.
- Lazdovica, K., et al. “Comparative wheat straw catalytic pyrolysis in the presence of zeolites, Pt/C, and Pd/C by using TGA-FTIR method.” Fuel Processing Technology, vol. 138, 2015, pp. 645–653., doi:10.1016/j.fuproc.2015.07.005.
- Dumitriu, Georgiana-Diana, et al. “Study of the potential use of mesoporous nanomaterials as fining agent to prevent protein haze in white wines and its impact in major volatile aroma compounds and polyols.” Food Chemistry, vol. 240, 2018, pp. 751–758., doi:10.1016/j.foodchem.2017.07.163.
- Dolbin, A. V., et al. “Quantum effects in the sorption kinetics of 4He by mesoporous materials.” Low Temperature Physics, vol. 42, no. 2, 2016, pp. 80–84., doi:10.1063/1.4941598.
- Jabbari-Hichri, Amira, et al. “Effect of aluminum sulfate addition on the thermal storage performance of mesoporous SBA-15 and MCM-41 materials.” Solar Energy Materials and Solar Cells, vol. 149, 2016, pp. 232–241., doi:10.1016/j.solmat.2016.01.033.
- Lazdovica, K., et al. “Catalytic pyrolysis of wheat bran for hydrocarbons production in the presence of zeolites and noble-Metals by using TGA-FTIR method.” Bioresource Technology, vol. 207, 2016, pp. 126–133., doi:10.1016/j.biortech.2016.01.117.
- Gignone, Andrea. “Ordered Mesoporous Silica for Drug Delivery in Topical Applications.” Tesi di dottorato, 2016, p. 136., doi:10.6092/polito/porto/2652565.
- Rios, Albertina Gonçalves. “Chromatographic Separation of Dyes in a Fixed Bed Adsorber.” LSRE – Laboratory of Separation and Reaction Engineering, 2017.
- Mazzeo, Nicolas. "Silici mesoporose come carrier di idrocortisone= Mesoporous silicas as hydrortisone carriers." PhD diss., Politecnico di Torino, 2018.
- Mazzeo, Nicolas. "Silici mesoporose come carrier di idrocortisone= Mesoporous silicas as hydrortisone carriers." PhD diss., Politecnico di Torino, 2018.
- Dumitriu, Georgiana-Diana, Nieves López de Lerma, Valeriu V. Cotea, and Rafael A. Peinado. "Application of Mesoporous Materials as Fining Agents for Pedro Ximénez Wines." (2018).
- El Roz, Ayman, Pascal Fongarland, and Mickael Capron. "Glycerol to Glyceraldehyde Oxidation Reaction Over Pt-Based Catalysts Under Base-Free Conditions." Frontiers in Chemistry 7 (2019): 156.
