-
Y-type Zeolites
Aug 19, 2019 | ACS MATERIAL LLCDealuminated ultrastable Y zeolites have increasingly attracted attention as good adsorbents and stable acid catalysts. These materials exhibit high thermal and hydrothermal stability, as well as higher catalytic activity than that of aluminum-rich synthesized Y zeolites. The ultrastable Y zeolite (USY) in fluid cracking catalysis is commonly stabilized by ion-exchange with rare earth (RE) cations. These rare earth ultrastable Y zeolites, also known as REUSY, demonstrate how rare earth exchange can provide much needed hydrothermal stability.
Introduction
Y zeolite (IZA structure, FAU type) is characterized by large, essentially spherical, internal cavities (supercages) linked tetrahedrally through pore openings of about 0.8 nm and defined by rings of twelve oxygen atoms.
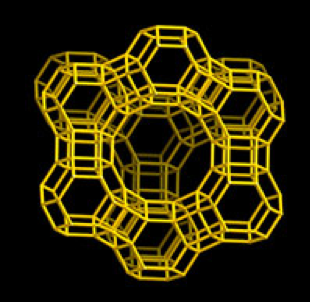
Figure 1. Structure of FAU type zeolite
The commercial synthesis of Y zeolite was claimed by Breck1 in 1964 after the first industrial manufacturing of A and X type zeolites by Milton.2,3 Zeolite NaY appeared to be topologically analogous with the type X aluminosilicate framework and the cubic unit cell of these alumosilicates was known to contain 192 (Si,Al) O4 tetrahedrons. Breck suggested that the change of X to Y modification occurs at a silicon to aluminum ratio of 1.5.4 Thus, Zeolites with Si/Al values lower and higher than this critical point characterize X and Y composition, respectively. Due to the instability of Al-rich samples in acids or water at elevated temperatures,5 Y samples above Si/Al ratio of 2.2 may be dealuminated in steam without having the framework collapse.6
Synthesis
(1) Synthesis of NaY Zeolite
The synthesis of aluminum-rich zeolites such as A, X, and Y types relies on the crystallization process of zeolites from their primary aluminosilicate gels. Properties of dealuminated Y samples such as crystallinity, silicon to aluminum ratio and secondary pore volume depend directly on the state of the parent NaY crystals and even on the starting gel as well. Typical compositions of synthesis precursors of aluminosilicate zeolites are as follows:
2.0 SiO2 : 1 Al2O3 : 3.4 Na2O : 170 H2O NaA
4.5 SiO2 : 1 Al2O3 : 6.3 Na2O : 280 H2O NaX
9.0 SiO2 : 1 Al2O3 : 3.0 Na2O : 120 H2O NaY
Final Si/Al ratios of 1, 1-1.2, and 2.2-3.0 are obtained respectively7.
Since zeolite Y can be directly synthesized with Si/Al ratios above 3, catalytically relevant materials with high Si/Al ratios must be prepared by post-synthetic removal of the framework aluminum. Furthermore, heating NH4Y in dry vs. wet air brings about different dealumination effects and structural characteristics 8.
(2) Synthesis of Ultrastable Y Zeolite
Ultrastable Y (USY) zeolite is an effective catalyst used for catalytic cracking in petroleum refining. This catalyst is prepared from ammonium Y zeolite by steaming at high temperatures above 773 K. From this process, aluminum cations are dislodged from the framework of the Y zeolite. Through this process, as the actual catalytic cracking occurs, the structure of the Y zeolite is simultaneously stabilized.9
(3) Synthesis of REUSY Zeolite
Among commercial fluid catalytic cracking (FCC) catalysts, REUSY, is one of the most commonly used. It is exchanged with mixed rare earth elements consisting predominantly of lanthanum and cerium. The synthesis of REUSY starts with ultrastable Y zeolite prepared as described in the previous section. Different rare earth-exchanged zeolites are then prepared by either (a) acid treatment of the ultrastable Y zeolite at a set pH, followed by rare earth exchange, or (b) exchanging the zeolite with a rare earth chloride solution of a certain acidity. Various types of Y-type series zeolites are available in powder and pellet forms on our ACS Material online store. Figure 2 below shows an SEM image of our Y-RE2 type REUSY.

Figure 2. SEMimage of ACS Material Y-RE2 (REUSY)
Applications
(1) Adsorber/ Catalyst Composites
Active ingredients in adsorber/catalyst composites on the basis of high-silica dealuminated Y zeolites are exclusively localized on the crystal surface while the bulk remains free for adsorption. Thus, high conversion of acetone is possible on H-dealuminated Y composites with Si/Al = 150, n-butane on a Ni-dealuminated or Pt-dealuminated Y zeolites.10 The most popular application of such composites is the purification of waste water in which pollutants such as halogenated hydrocarbons must be removed up to a few nanograms. In this process, pollutants are first separated by adsorption from a liquid phase, and then decomposed by chemical reaction by oxidation with hydrogen peroxide as an example.
Wolfgang Lutz et al. tested the decomposition of p-chloro-phenol in water on Pt-dealuminated Y samples as compared to Fenton’s reagent (FeSO4).11 The zeolite was loaded by 5mg, 12mg, 23mg, and 47mg of Pt per g composite. The best effect was obtained with 5mg/g because of an optimized activation of the admixed hydrogen peroxide.
(2) FCC Catalyst
Zeolite Y has been the primary active component of fluid catalytic cracking (FCC) catalysts since its first commercial introduction about 50 years ago.12,13,14 This is mainly because of the unique combination of a few important properties of zeolite Y: (1) high surface area and relatively large pores (~7.4 Å in diameter); (2) strong Brønsted acidity; (3) excellent thermal and hydrothermal stability; and (4) low cost. When other zeolites are used as active components in FCC catalysts, their catalytic performance (activity and product selectivity) cannot compare with that of zeolite Y due to its specialized properties.
Conclusion
Zeolite Y is widely used in in catalytic cracking due to its specialized properties such as structure stability, hydrophobicity and catalytic activity. The ultrastable Y zeolite in fluid cracking catalysts is commonly stabilized by ion-exchange with rare earth (RE) cations and the RE-exchange provides hydrothermal stability to the zeolite. Thus, REUSY, is a prime example of how rare earth exchange can provide hydrothermal stability by improving surface area retention and inhibiting dealumination, resulting in greater preservation of acid sites.
ACS Material Products:
References
1. D. W. Breck, “Crystalline zeolite Y,” U.S. Patent 3130007, 1964.
2. R. M. Milton, “Molecular sieve adsorbents,” U.S. Patent 2882243, 1959.
3. R. M. Milton, “Molecular sieve adsorbents,” U.S. Patent 2882244, 1959.
4. D. W. Breck, Zeolite Molecular Sieves, John Wiley & Sons, New York, NY, USA, 1974.
5. E. M. Flanigan, “Molecular sieve zeolite technology: the first twenty-five year,” in Proceedings of the 5th International Conference on Zeolites, pp. 760-780, Naples, Italy, June 1980.
6. C. W. McDaniel and P. K. Maher, in Molecular Sieves, p. 186, Society of Chemical Industry, London, UK, 1968.
7. D. W. Breck and E. M. Flanigan, “Synthesis and properties of union carbide zeolites L, X and Y,” in Molecular Sieves, pp. 47-60, Society of Chemical Industry, London, UK, 1968
8. J. W. Ward, “Termal decomposition of ammonium Y zeolite,” Journal of Catalysis, vol. 27, pp. 157-161, 1972.
9. Scherzer, J., Bass, J. L., J. Catal.,46, 100 (1977)
10. W. Lutz, D. L. Hoang, G. Lischke, and B. Parlitz, “Extraframework aluminium in DAY zeolites as carrier for catalytic ingredients,” in Proceedings of the 3rd Polish-German Zeolite Colloquium,M. Rozwadowski, Ed., pp. 205–214, Nicolas Copernicus University Press, 1998.
11. W. Lutz, R. Bertram, W. Wieker, and M. Jank, “Adsorber- Katalysator-Komposite für Umweltprozesse,” in Neue Entwicklungen zur adsorptiven Gas- undWasserreinigung, W. Henschel, Ed., vol. 859 of Freiberger Forschungshefte A: Verfahrenstechnik/ Umwelttechnik, p. 256, Bergakademie Freiberg, Freiberg, Germany, 2000.
12. A. F. Masters and T. Maschmeyer, Microporous Mesoporous Mater., 2011, 142, 423.
13. T. F. Degnan, Top. Catal., 2000, 13, 349.
14. C. Matínez and A. Corma, Coord. Chem. Rev., 2011, 255, 1558
FAQ
Q. What is zeolite Y used for?
A. Y-type zeolites are for carbon dioxide adsorption applications due to their high stability and excellent ion exchange. It is also used in composites where pollutants are absorbed and later chemically break down. The high thermal stability and large pore structure make this perfect for industrial catalysts.
Q. Is Zeolite Good for You?
A. Although zeolite supports detoxification and digestive balance by trapping toxins and heavy metals in the gut, scientific evidence in humans is limited, and its benefits are still unproven.
Q. How to Use Zeolite?
A. Zeolite is commonly available in capsule, powder, and liquid forms. Sometimes, it comes in drop form, which you take with diluted water on an empty stomach. However, never take without consulting a medical expert.
Q. Is Zeolite Safe for Cats?
A. Some particular forms of natural zeolites, like clinoptilolite, are allowed to be used in animal feed to improve digestion and reduce ammonia, but in a specific quantity.
Q. How Much Zeolite Should I Take?
A. The dosage of zeolite varies widely between brands, but the clinical studies haven’t established a safe or effective amount. So, consult a doctor and follow the manufacturer’s label instructions.
Q. Can You Take Zeolite with Other Medications?
A. It’s better to take zeolites at least 2-4 hours apart from any medication, according to the experts.
Q. How to Use Zeolite for Detox?
A. No research and studies have proven the use of zeolite for detoxification of the body; however, people consume it by mixing it in water or juice. Also, you can take it in capsule form for short periods.
Q. How Does Zeolite Work in the Body?
A. The zeolite pore structure is a charged framework that attracts positively charged ions like toxins and heavy metals, cadmium, chromium, and magnesium. And, these are carried out of the body through natural elimination.