-
Titanium Dioxide
Feb 28, 2019 | ACS MATERIAL LLCTiO2 nanomaterial is notable for its large specific surface area and unique chemical, physical, optical and electronic properties. It is also non-toxic and environmentally friendly in nature and demonstrates excellent biocompatibility and stability. These unique properties make it useful for a wide array of applications including photocatalysis, gas and humidity sensors, water treatment, solar cells, photochemical cells and protective coatings on optical elements. TiO2 is also widely exploited as a pigment providing whiteness and opacity given by its high brightness and very high refractive index. As a pigment TiO2 is used to produce paints, coatings, plastics, papers, inks, foods, toothpastes, sunscreens and various other cosmetics.
Introduction
Titanium dioxide (TiO2) was discovered by Honda and Fujishima in the year 1971 and was first used for the photooxidation of water.1 TiO2 has since been widely applied in photocatalysis for environmental cleanup, solar cells, clean H2 energy production and antibacterial purposes.2 In nature, TiO2 has four main polymorphs including anatase, rutile, brookite and TiO2(B). All four types of TiO2 consist of TiO6 octahedra but differ in the distortion of the octahedron units with shared edges and corners occurring in different configurations (Figure 1). For anatase, the octahedra are arranged in zigzag chains along {221} sharing four edges. In rutile, octahedra share only two edges and connect in linear chains parallel to {001};3 while in brookite both corners and edges are connected.4 Given that TiO2(B) is mainly formed from layered titanates makes the structure of TiO2(B) similar to that of its layered precursor composed of corrugated sheets with TiO6 octahedra sharing edges and corners.5 These differences in lattice structures cause variations in mass densities and electronic band structures in the different phase forms of TiO2.
Rutile is the most thermodynamically stable of the phases, while anatase, brookite, and TiO2(B) are metastable. Rutile can be easily obtained by annealing the other three polymorphs at elevated temperatures. This makes the transition process from TiO2(B) to rutile similar to that of brookite to rutile, following the TiO2(B) → anatase → rutile route.6,7 Both anatase and rutile have been extensively studied for photocatalytic applications. Brookite and TiO2(B) are not commonly available, so their applications are not described here.
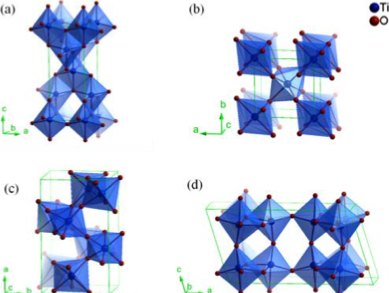
Figure 1. Crystalline structures of TiO2 in different phases: (a)
anatase, (b) rutile, (c) brookite and (d) TiO2(B).8The unique properties of TiO2 strongly depend on the physical characteristics of TiO2 including crystallization, grain size, morphology, specific surface area, surface state and porosity. Various physical forms of TiO2 can be synthesized, such as nanoparticles, nanowires, nanorods, nanotubes and nanofibers.

Figure 2. Image of ACS Material TiO2 Nanoparticles
Synthesis
Several preparation methods can be used to synthesize TiO2 nanoparticles including sol-gel, hydrothermal, solvothermal, microemulsion and vapor deposition methods.
TiO2 nanoparticles (Figure 2) as available on our ACS Material online store are commercial Degussa P25 TiO2, which has a 3:1 combination ratio of anatase to rutile phases. Their average crystal size is 10-40 nm.9
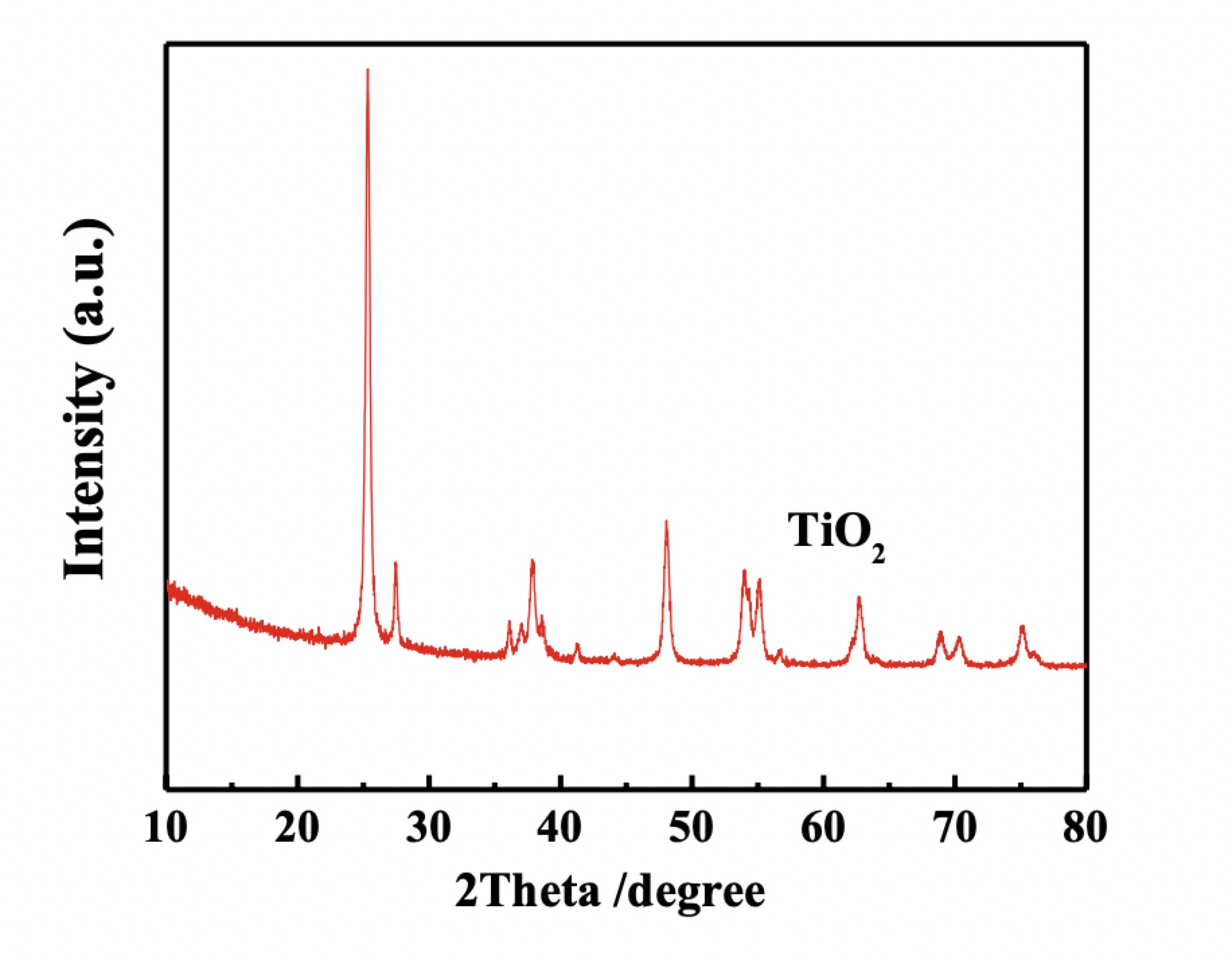
Figure 3. XRD pattern of TiO2 Nanoparticles
The XRD pattern of our P25 TiO2 as shown in Figure 3 above indicates both anatase and rutile phases, which correspond to the patterns in JCPDS Nos. 88-1175 (anatase) and 84-1286 (rutile), respectively. The anatase phase pattern shows peaks at 25.2, 37.7, 48.0, 53.8, 55.0, and 62.6° that correspond to (101), (004), (200), (105), (211) and (204) planes respectively. Furthermore, the rutile phase pattern peaks at 27.4, 36.0, 41.2, 44.0, and 56.6° corresponding to the (110), (101), (111), (210) and (220) planes, respectively.7
The TEM images presented in Figure 4 show the general morphology of our ACS Material TiO2 nanoparticles. They are most often roughly spherically shaped and some of them appear to be facetted. The particles are visibly arranged in a chain-like configuration.
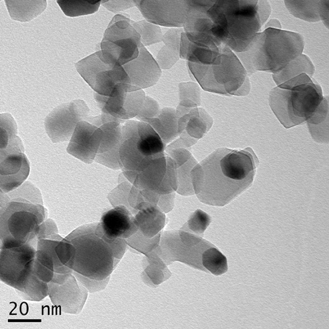
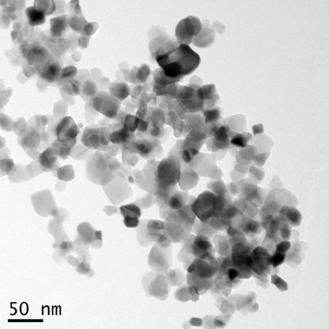
Figure 4. TEM images of TiO2 Nanoparticles
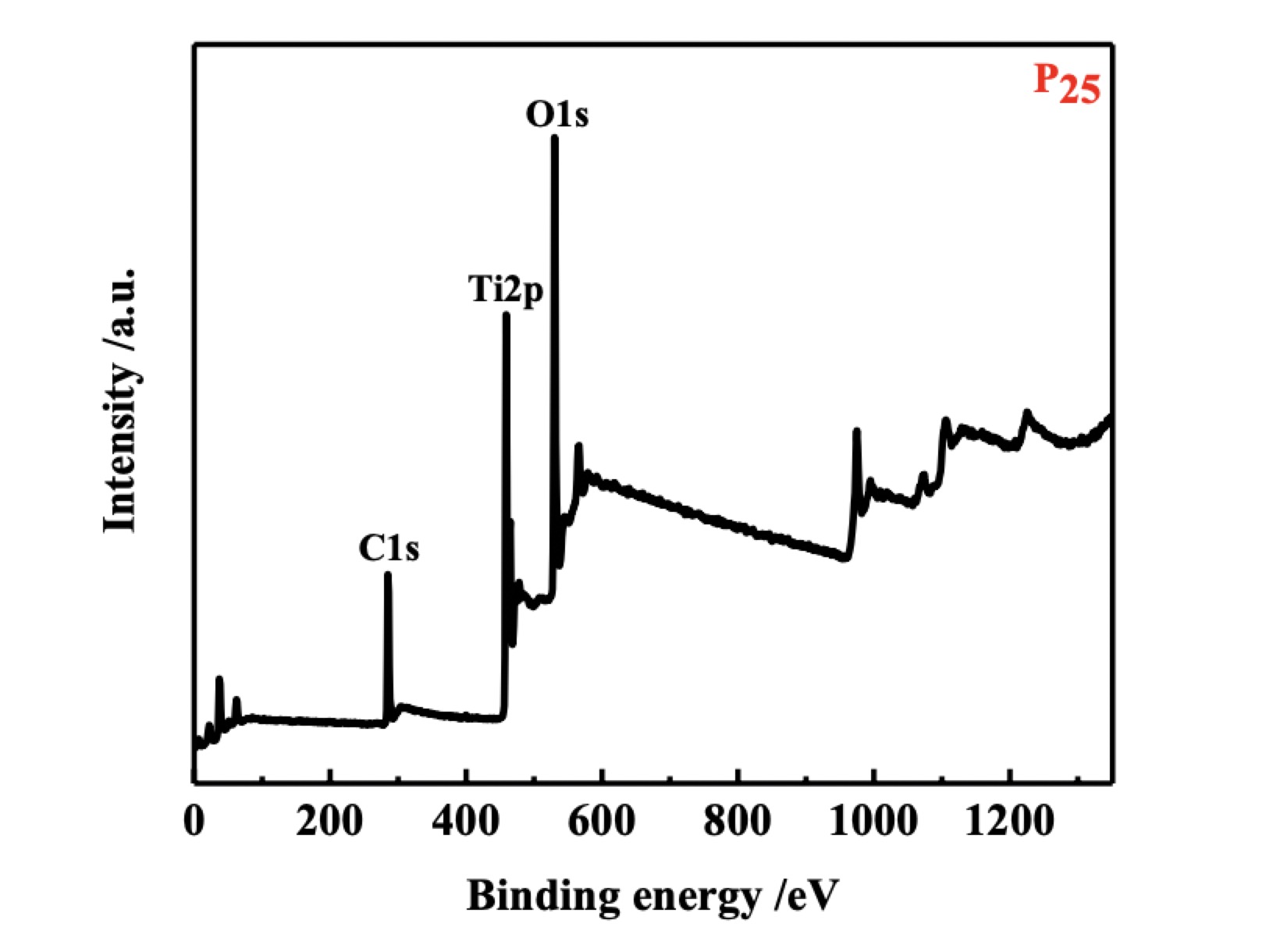
Figure 5. XPS spectra of TiO2 Nanoparticles
Figure 5 shows the full spectra of P25 with peaks at 283.7, 458.7 and 530.8 eV corresponding to C1s, Ti2p and O1s peaks, clearly revealing the presence of C, Ti and O. The carbon peak is ascribed to the carbon tape used for the XPS measurements. Therefore, if the carbon peak is excluded, the full spectra indicate only the Ti-O binding states.
Applications
Over the past few decades, TiO2 has been found to be quite useful in various applications such as photovoltaics, photocatalysis and sensors.10-11 TiO2 possesses nontoxic, biocompatible, photocorrosion free and cost-effective advantages..12 Additionally, a variety of nanostructures enables them to achieve extraordinarily large surface area as well as unique chemical, physical and electronic properties.
Photocatalytic Applications
The most extensive research on TiO2 has gone to using it as a solar energy conversion material, which is mainly investigated on anatase and rutile. To this day, various research works have been carried out using TiO2 as a model semiconductor photocatalyst to produce H2 from water splitting, biomass reforming, industrial waste reforming and to produce carbon-based solar fuels via CO2 photoreduction. Anatase and rutile TiO2 have different densities and electronic band structures, leading to different band gaps (for bulk materials: anatase 3.20 eV corresponding to 384 nm and rutile 3.02 eV corresponding to 410 nm).
One of the main drawbacks of TiO2 is its ability to absorb only UV region light. Much effort has been devoted to extending its light absorption to the visible region. Some efficient strategies include introducing other elements into TiO2, using special preparation methods and surface sensitization, which refers to the application of other visible light active materials as light harvesters to sensitize TiO2.
Photovoltaic (PV) Applications
Among the renewable energy technologies currently available including hydro, solar, wind, geothermal heat and biomass, PV technology, which converts solar energy into electricity is expected to be the most promising strategy for supplying sustainable energy. Solar photovoltaic technology is now regarded as the third most important renewable energy source in terms of globally installed capacity. By the end of the year 2012 the world’s cumulative solar PV capacity surpassed the 100 GW milestone.13
The application of TiO2 nanomaterials in photovoltaic devices include dye-sensitized solar cells (DSCS), polymer-inorganic hybrid solar cells, quantum dot-sensitized solar cells, inorganic solid-state solar cells and perovskite solar cells. For example, the nanocrystalline morphology of TiO2 film is critical for the efficient operation of DSCs. A major breakthrough for DSCs occurred in 1991 by using a mesoporous TiO2 electrode with high internal surface area to support the monolayer of a sensitizer, which enhanced its light harvesting ability as well as the photocurrent of the device. 14
Sensor Applications
Many novel TiO2 nanomaterials with new compositions and structures have been reported for use in various sensors. On the basis of different sensing targets or measurement principles, sensors can be referred to as gas sensors, optical sensors, electric sensors, environmental sensors, biosensors, etc. Three typical TiO2-based sensors are: (1) TiO2-based gas sensor for detecting gas or volatile chemicals; (2) TiO2-based chemical oxygen demand (COD) sensor for detecting soluble organics in water environment; and (3) TiO2-based biosensor for detecting biological substances. These sensors are categorized according to the target substance, the integrated science within and the sensing performance. For example, TiO2 has strong capabilities in oxidizing organic matters under appropriate illumination, which makes TiO2-based photocatalytic /photoelectrocatalytic sensing techniques highly attractive in the field of environmental monitoring and environmental protection.15
Conclusion
TiO2 nanomaterials possess large specific surface area and unique properties in chemical, physical, optical, electronic and photocatalytic fields. Moreover, they are nontoxic, environmentally friendly and demonstrate excellent biocompatibility and stability. These important characteristics allow TiO2 nanomaterials to be adopted in a wide range of photocatalytic, photovoltaic and sensor applications.
ACS Material Products:
References
1. Fujishima A. and Honda K., Bull. Chem. Soc. Jpn., 1971, 44, 1148-1150.
2. Chen X. B. and Mao S. S., Chem. Rev., 2007, 107, 2891-2959.
3. Gopal, M., Chan, W. J. M., DeJonghe, L. C. J. Mater. Sci. 1997, 32, 6001
4. Carp, O., Huisman, C. L., Reller, A. Prog. Solid State Chem. 2004, 32, 33
5. Feist, T. P., Davies, P. K. J. Solid State Chem. 1992, 101, 275
6. Li, W., Bai, Y., Liu, C., Yang, Z. H., Feng, X., Lu, X. H., van der Laak, N. K. and Chan, K. Y. Environ. Sci. Technol. 2009, 43, 5423.
7. Jitputti, J., Suzuki, Y., Yoshikawa, S. Catal. Commun. 2008, 9, 1265
8. Ma Y., Wang X. L., Jia Y. S., Chen X. B., Han H. X., and Li C., Chem. Rev. 2014, 114, 9987-10043.
9. Han E. J., Vijayarangamuthu K., Youn J. S., Park Y. K., Jung S. C., Jeon K. J., Catal. Today 2017, 8, 57.
10. Konstantinou I. K., Albanis T. A. Appl. Catal., B 2004, 49, 1.
11. Ghicov A., Schmuki P. Chem. Commun. 2009, 20, 2791
12. Bai Y., Mora-Sero I., De Angelis F., Bisquert J. and Wang P., Chem. Rev. 2014, 114, 10095.
13. O’Regan, B.; Gratzel, M. Nature 1991, 353, 737
14. Sun Z., Kim J. H., Zhao Y., Bijarbooneh F., Malgras V., Lee Y., Kang Y. M., Dou S. X., J. Am. Chem. Soc. 2011, 133, 19314.