-
Mordenite Zeolite
Oct 24, 2018 | ACS MATERIAL LLCMordenite (MOR) is a high-silica molecular sieve with two pore channels. It is synthesized by the hydrothermal method and has been effectively used in the adsorption and separation of gas or liquid mixtures involving acidic components. As a catalyst, mordenite zeolite is used in various important industrial reactions like hydrocracking, hydro-isomerization, alkylation, reforming and cracking.
Introduction
Mordeniteis a silica-rich, large-pore zeolite that occurs readily in nature. Although mordenite occurs in nature as a mineral, synthetic mordenites are better suited in terms of purity for meeting stringent requirements for adsorption and catalytic processes. The first claim to the synthesis of mordenite was by R.J. Leonard in 1927.1Mordenite with two-dimensional pores was the structure determined by W.M. Meier in 19612.The pore system of mordenite consists of main channels of 6.5 × 7.0 Å, which are connected by tortuous pores of 2.6 × 5.7 Å that form the so-called “side pockets”.3 The unit cell of the sodium form of mordenite has the following dimensions: a = 18.13Å, b = 20.49 Å, and C = 7.52 Å, and its chemical constitution is represented by (Na2O)4.(A12O3)4.(SiO2)40.24H2O.4
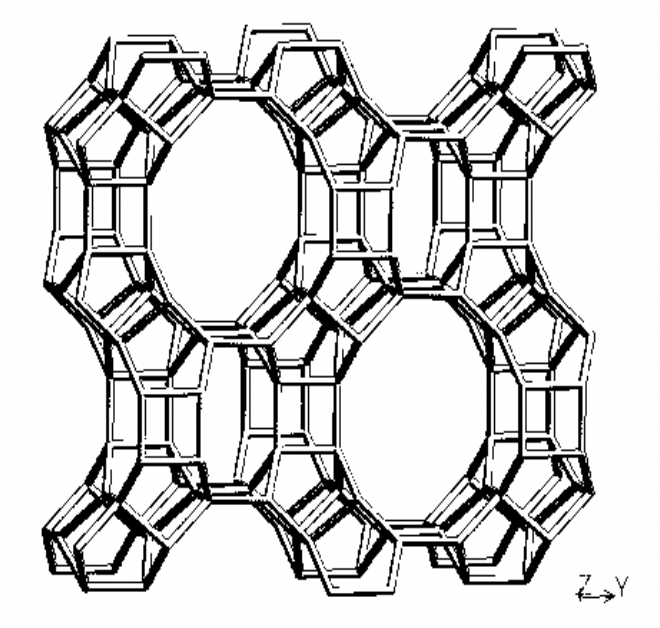
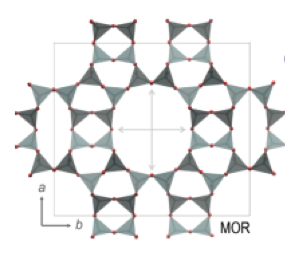
Figure 1. Framework of MOR zeolite.2The MOR framework defines one-dimensional channels (12-rings, 6.5× 7.0 Å) parallel to the c-axis.
Mordenite is widely used in catalysis and in separation andpurification because of its uniform, small pore size, high internal surface area, flexible framework, and controlled chemistry.
Synthesis
The synthesis of mordenite is generally prepared within narrow limits of SiO2/Al2O3 ratios. Most studies on the direct synthesis are reported with SiO2/Al2O3 ratios ranging from 9 to 20.5 When organic materials are added during synthesis, greater ratios of SiO2/Al2O3 can be obtained. For the synthesis of mordenite, silica has been used in the form of sodium silicate and silica gel. It has been reported that starting with aluminosilicate gel rather than individual oxides facilitates better synthesis of mordenite.
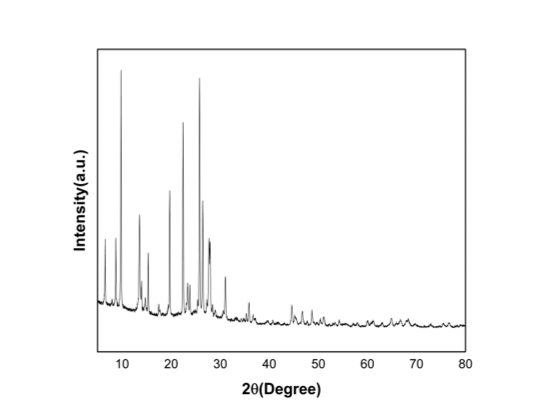
Figure 2. XRD spectra of Mordenite Zeolite
The sodium aluminosilicate is prepared by mixing sodium aluminate with sodium silicate. If more silica is required, the appropriate quantity of silica gel is added to the sodium silicate before making the gel.
The hot solution of the starting mixture is kept in the autoclave for the hydrothermal reaction. At the end of each run conducted for a specific period of time, the product is filtered and subsequently washed with hot distilled water until the pH of the filtrate reaches 7.6 The XRD patterns of mordenite samples display sharp reflection peaks between 5◦ to 50◦(Figure 2). Mordenite zeolite with a SiO2/Al2O3 ratio of about 20 is available on our ACS Material online store.
Applications
Due to its high thermal and acid stability, mordenite has been used as a catalyst for important reactions such as hydrocracking, hydroisomerization, alkylation, reforming, dewaxing, and the production of dimethylamines.7 These reactions preferably take place onthe surface of the main channel due to the range of channel sizes within the mordenite zeolite structure. Mordenite has also been used in the adsorption and separation of gas and liquid mixtures.8 In addition, mordenite has been considered for applications in semiconductors, chemical sensors, and nonlinear optics.9 The properties of mordenite can be modified by adjusting different synthetic parameters such as the source of aluminum, the presence of seeds, the use of low temperatures, longer crystallization times, and different silica to alumina ratios. It is worth noting that mordenite-type zeolites with high silica contents are preferred when the reactions take place at high temperatures and in particular where acidic components are involved.
Conclusion
Mordenite is widely used in catalysis, separation and purification because of its uniform and small pore size, high internal surface area, flexible framework, and controlled chemistry. The major drawbacks of mordenite zeolites in general are the limited sizes of the channels and cavities and the lack of interconnectivity. This imposes diffusional limitations on reactions, limiting their activity, selectivity and stability. Various strategies have been developed to synthesize mesoporous mordenite zeolites to enhance pore accessibility and to synthesize smaller crystals of zeolites, which will have larger surface areas and less diffusional limitations.
ACS Material Products:
References
1. LeonardR J.The hydrothermal alteration of certain silicate minerals. Economic Geology, 1927(1):18-43.
2. Slinkin A A, Loktev M I, Klyachko A L, et al. Study of the ESR method of the nature of the redox centers of mordenites in reactions of formation of cation radicals in the absorption of aromatic hydrocarbons. Bulletin of the Academy of Sciences of the Ussr Division of Chemical Science, 1975, 24 (5): 936-941.
3. Xu C, Guo Y, Xiao Q, et al. Synthesis and characterization of large, pure mordenite crystals. Journal of Porous Materials, 2012, 19 (5): 847-852.
4. Hincapie B O, Garces L J, Zhang Q, et al. Synthesis of mordenite nanocrystals. Microporous & Mesoporous Materials, 2004, 67 (1): 19-26.
5. F. Haimidi, R. Dutartre, F. diRenzo, A. Bengueddach, F. Fajula,in: Proceedings of the 12th International Zeolite Conference 3 (1999) 1803.
6. Shaikh A A, Joshi P N, Jacob N E, et al. Direct hydrothermal crystallization of high-silica large-port mordenite. Zeolites, 1993, 13 (7): 511-517.
7. Bajpai P K, Rao M S, Gokhale K V G K. Synthesis of mordenite type zeolite. Zeolites, 1986, 6 (1): 2-8.
8. Shao C, Kim H Y, Li X, et al. Synthesis of high-silica-content mordenite with different SiO2/Al2O3ratios by using benzene-1,2-diol as additives. Materials Letters, 2002, 56 (1): 24-29.
9. Gilbert J E, Mosset A. Large crystals of mordenite and MFI zeolites. Materials Research Bulletin, 1998, 33 (7): 997-1003.