-
Introduction to zeolites
Jul 15, 2018 | ACS MATERIAL LLCZeolites, which are also called molecular sieves, are crystalline microporous materials formed primarily by SiO4 and AlO4 corner-sharing tetrahedral building units that form three-dimensional (3D) frameworks with well-defined channels and cavities of molecular dimensions. The void space within the crystal allows zeolites to discriminate molecules based on their size or geometric shape. With variable chemical compositions and unique pore topologies, zeolites have been utilized in a wide range of industrial applications such as adsorption/separation, ion-exchange processes and also as catalysts in the processes of oil refining and fine chemical synthesis.
Introduction
Zeolites, which are found as minerals in nature, are an important class of inorganic microporous crystalline material whose oxide-based network is composed of corner-sharing TO4 atoms, where T refers to a tetrahedral atom, most commonly Si and Al. Since their discovery, zeolites have found widespread applications in many different areas, especially in catalysis, adsorption/separation, and ion-exchange processes1. Apart from these traditional uses of zeolites that have made a tremendous economic impact on different sectors of the chemical industry in the petrochemical sector, new uses of zeolitic materials are emerging for applications such as luminescence, electricity, magnetism, medicine, and microelectronics2. The ability for zeolites to be used in such a wide range of applications is directly associated with two particular characteristics of zeolites: (1) their unique microporous structure, with porous systems of channels and/or cavities of molecular dimensions which allow them to behave as molecular sieves or as shape selective catalysts, and (2) their versatile range of compositions of the oxide networks that can build up the different frameworks, most commonly Si and Al, but also extended to a large variety of other tetrahedral atoms such as B, Ge, P, and Ga. Oxide composition determines important properties of the zeolite such as concentration, strength of catalytic sites, and polar (hydrophilic/hydrophobic) character of the channel surface. Therefore, in order to optimize the zeolite, it is fundamental to take careful control of these two characteristics of zeolite materials, their porous network and their chemical composition.
Synthesis
Synthetic zeolites were initially made entirely from inorganic reagents and many experiments were performed to determine the effects of various alkali and alkali earth metals. A major advancement in zeolite synthesis came in 1961 when Professor R.M. Barrer, of Imperial College London, began experimenting with substituting part of the alkali and alkali earth cations with organic cations such as tetramethylammonium (TMA+)3. Using TMA+, he was able to synthesize several of the zeolites we use today.


Figure 1. SEM image of ACS Material Mesoporous Silica Molecular Sieve SBA-15
Chemists at the Mobil Oil Company also began to utilize organic cations in zeolite syntheses, and in 1967 they became the first research group to make new zeolite structures with organic cations, which are also referred to as templates or structure-directing agents (SDAs). Even today, the use of new SDAs remains the primary strategy for discovering new zeolite structures. As quoted from reference4, “templating is the phenomenon occurring during either the gelation or the nucleation process whereby the organic molecule organizes oxide tetrahedra into a particular geometric topology around itself and thus provides the initial building block for a particular structure type.”
Synthesis methods for zeolites are numerous, including hydrothermal/solvothermal/ionothermal approaches, and sometimes the syntheses are also performed through dry-gel, microwave-assisted, or solvent free methods. SDAs are indispensable in the synthesis process of zeolites.
Applications
Zeolite micropores are of molecular size, which gives them adsorption (Beta Zeolite and Y-Type Series Zeolite), catalytic (SBA-15, SBA-16, MCM-41, Al-MCM-41, MCM-48, FDU-12, ZSM-5, ZSM-11, ZSM-22, ZSM-35, SAPO-11, SAPO-34, TS-1, SSZ-13 and KIT-6), and ion-exchange (Beta Zeolite) properties, which are of paramount importance in the industrial chemical field. Moreover, interest is growing in the study of new zeolite applications related to process intensification, green chemistry, hybrid materials, medicine, animal food uses, optical-and electrical-based applications, multifunctional fabrics, and nanotechnology. Several applications are described in the following sections.
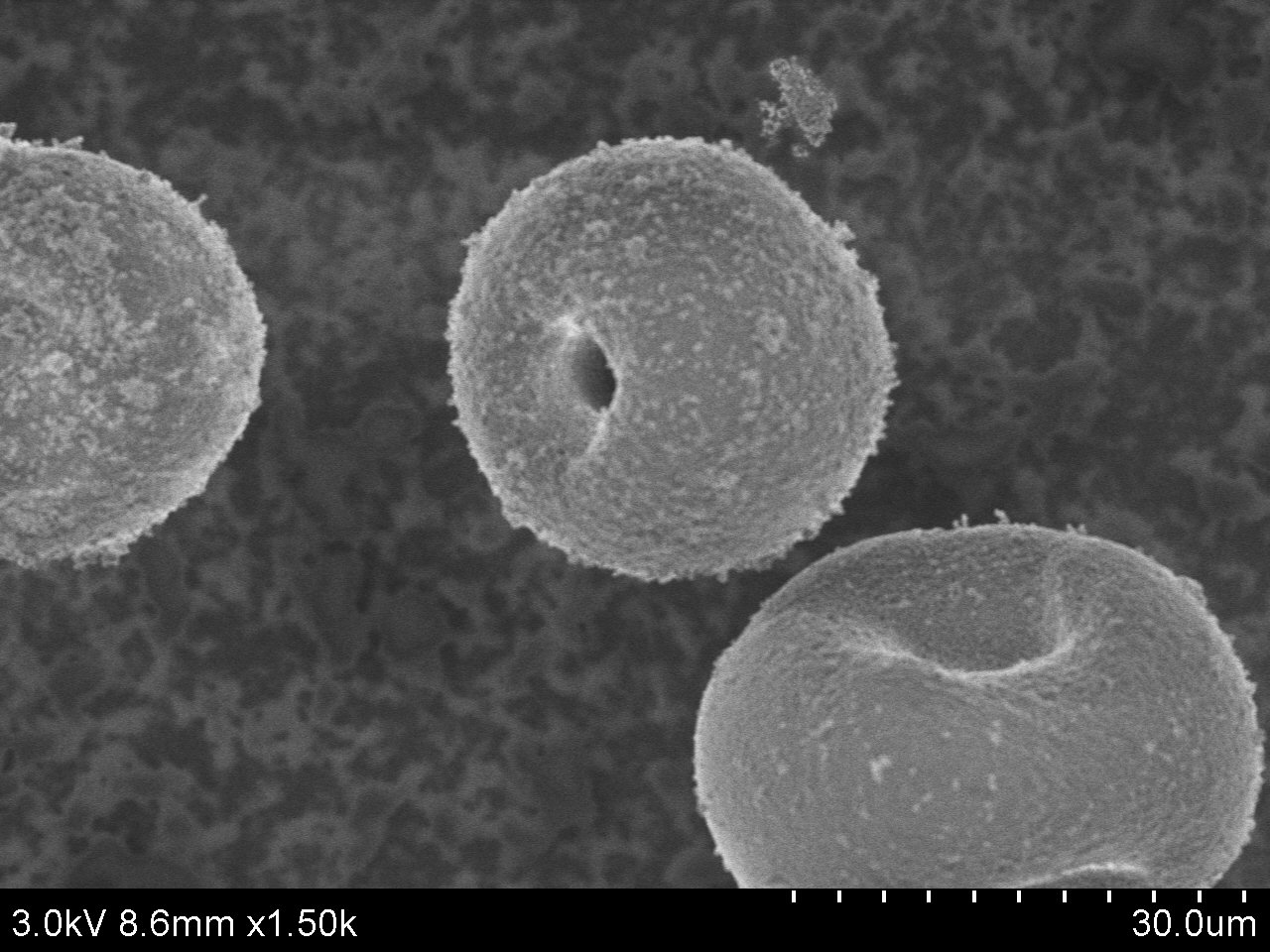
Figure 2. SEM image of ACS Material Molecular Sieve TS-1 (Type B)
Advanced Separation Technologies
The most fundamental consideration regarding the adsorption of chemical species by zeolites is molecular sieving. The micropore size determines the accessibility of adsorbate molecules to the internal adsorption surface. Species with a kinetic diameter too large to pass through zeolite pores are effectively ‘‘sieved’’. This ‘‘sieve’’ effect can be utilized to separate molecules by size and shape. The selectivity of zeolites for a particular adsorbate depends on steric effects, magnetic susceptibility, and polarizability of the molecules.
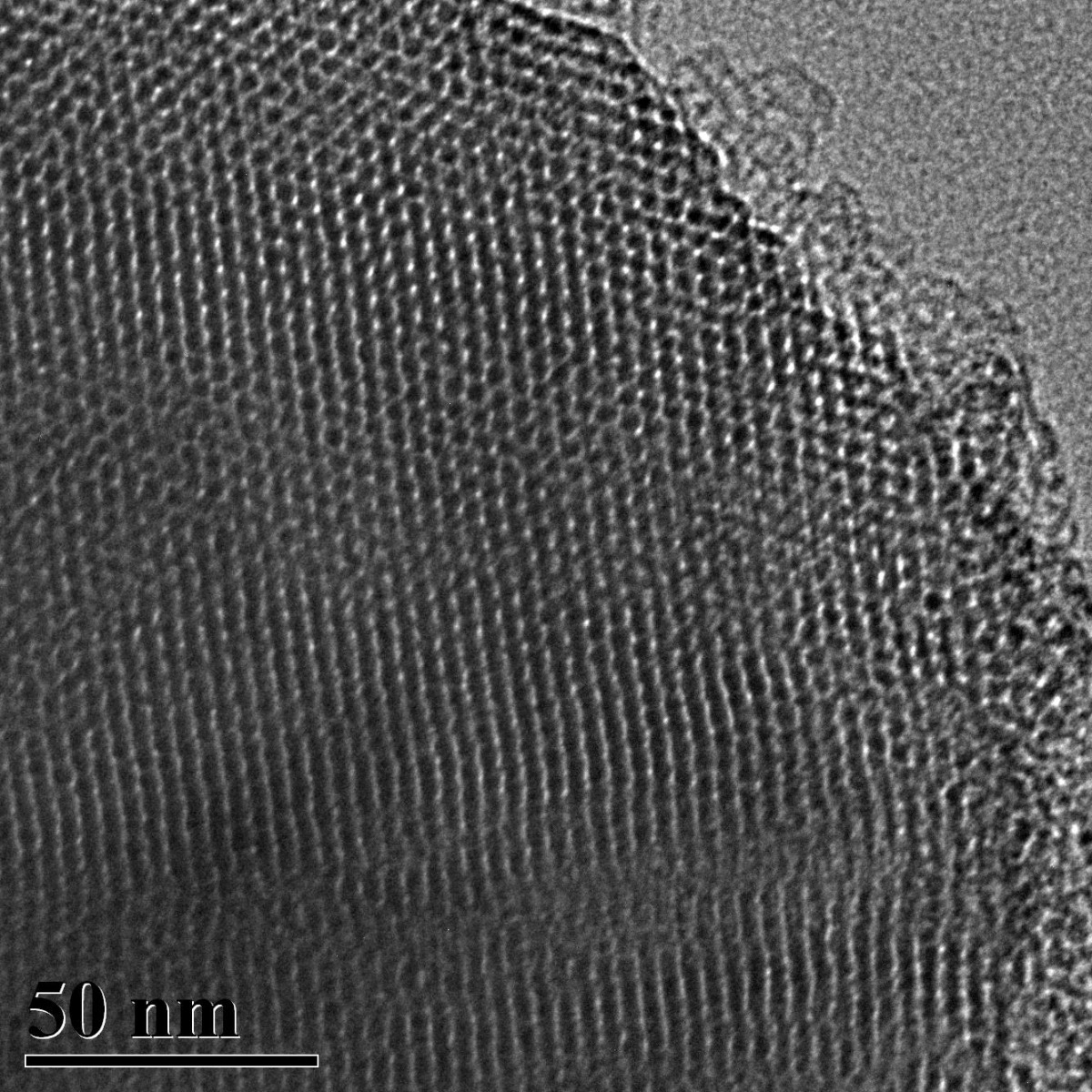
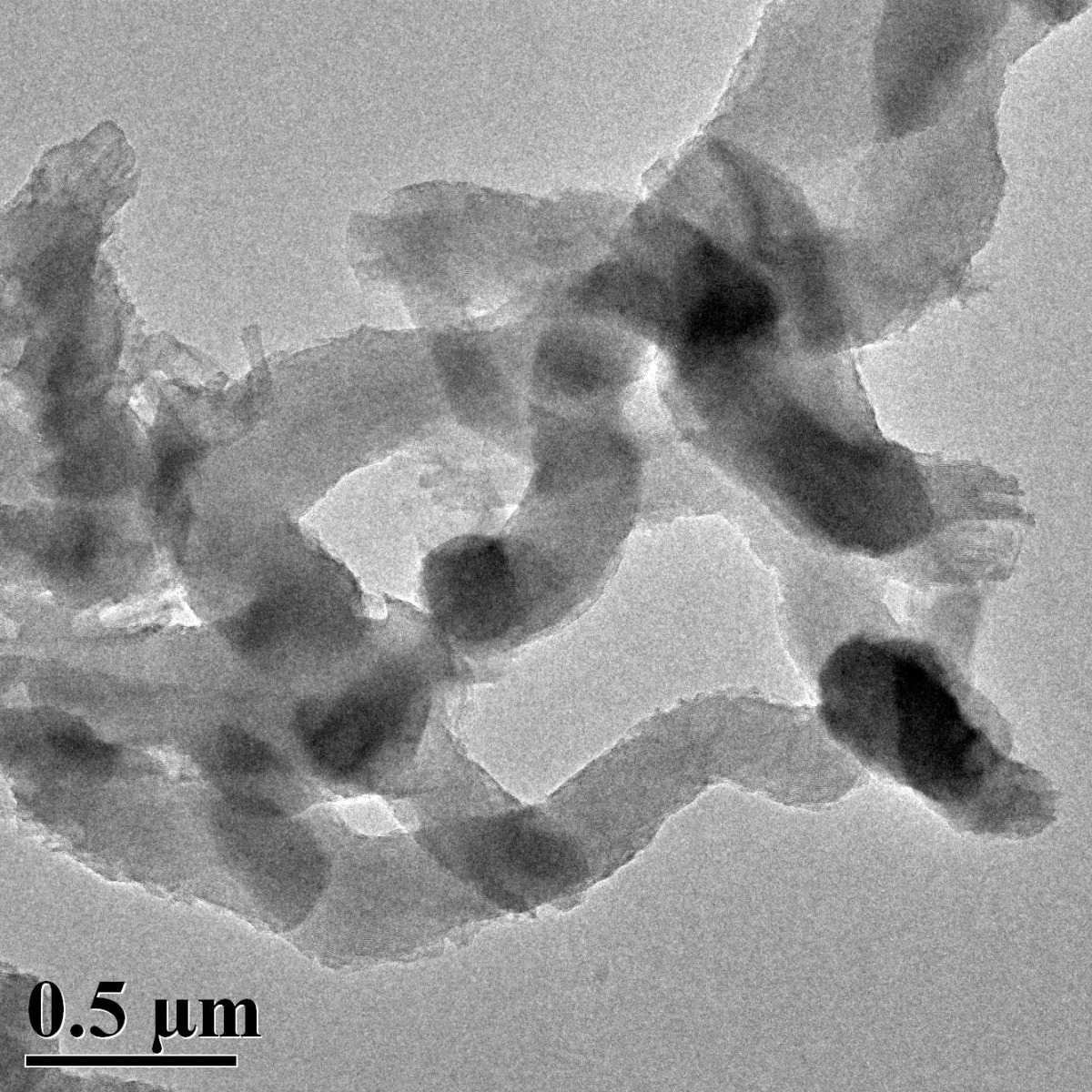
Figure 3. SEM image of ACS Material Molecular Sieve MCM-41 (Type A)
Zeolites are used in many separations and purification applications in several industrial fields such as petroleum refining processes, petrochemicals, natural gas treatment, industrial gas production and purification, specialty chemicals, and pharmaceuticals. Adsorption on zeolites also plays a relevant role in environmental waste reduction applications (i.e., recovery of solvents from industrial off-gases, radioactive waste management, and making builders for phosphate-free laundry detergents). Representative commercial adsorption separations performed with zeolites are reported in
Zeolites as Catalysts for the Synthesis of Fine Chemicals
Zeolites are crystalline microporous materials that are widely used in refining and for the production of chemicals and petrochemicals5. The merits of these materials as solid acid or basic catalysts have been extensively discussed in the literature. Particularly, acid zeolites have been widely used as catalysts in refining and petrochemical industries because of their excellent catalytic behavior, allowing for the replacement of hazardous acids, the reduction of salts and other waste products, and the prevention of plant corrosion.
In the past years, the amount of work on the use of acidic zeolites as catalysts for the synthesis of chemical intermediates and fine chemicals has increased considerably. The variety of pore topologies and pore dimensions within the zeolite materials along with the possibility to tune their acidity (or basicity) and the possibility to regenerate them make these materials attractive heterogeneous catalysts for the synthesis of fine chemicals.
Zeolites and Molecular Sieves in Fuel Cell Applications
Fuel cells promise clean and efficient energy generation for stationary, mobile, and portable applications6. Striving for enhanced performance, zeolites and mesoporous materials are increasingly used in fuel cells. They have been used to increase proton transport, decrease fuel crossover and improve water management in the electrolyte membrane. They serve as electrodes and electrocatalysts in fuel cells and are also employed in fuel conversion, reforming, and storage. The contributions of zeolites and molecular sieves in fuel cell research are mainly divided into three sections: (1) zeolites in electrolyte membranes, (2) zeolites in fuel cell electrocatalysis, and (3) zeolites in fuel processing for fuel cells.
Conclusion
The most important active sites of zeolite catalysts, their nature, and chemical behavior depend greatly on the structure type and the chemical composition of the framework. It is reported that the impact of zeolites in science and technology in the last 50 years has no precedents in the field of materials and catalysis. Almost 200 different structural types of zeolites and numerous excellent scientific papers about zeolites have revolutionized the petrochemical industry. In addition, other porous materials have emerged including mesoporous materials, hierarchic systems, metal-organic frameworks (cationic-periodic polymers) and mesoporous organosilicas. All these materials have substantially increased the interesting properties of novel porous materials. The possibilities for new and exciting achievements in zeolite research are seemingly endless as zeolite developments have the potential to reach as far as our imaginations can guide it.
ACS Material Products:
References
1. Lough WJ, Wainer IW (eds) (2002) Chirality in natural and applied science. CRC Press. ISBN 0-632-05435-2
2. Cahn RS, et al. “Specification of Molecular Chirality.”Angewandte Chemie International Edition, vol. 5, no. 4, 2010, pp.385-415.,doi: 10.1002/anie.196605111.
3. Barrer RM,et al. 201. “Hydrothermal chemistry of the silicates. Part IX. nitrogenous aluminosilicates.” Journal of the Chemical Society, 1961, 971-982., doi: 10.1039/jr9610000971.
4. Davis, Mark. “Zeolites from a materials chemistry perspective.” Chemistry of Materials, vol. 26, no. 1, 2013,pp. 239–245., doi: 10.1021/cm401914u.
5. R.A. Sheldon,et al. “Heterogeneous catalytic transformations for environmentally friendly production.” Applied Catalysis A General,vol. 189, no. 2, 1999,pp. 163-183.,doi:10.1016/S0926-860X(99)00274-4.
6. Yuyan, Shao, et al.“Proton exchange membrane fuel cell from low temperature to high temperature: Material challenges.”Journal of Power Sources, vol. 167, no. 2, 2007,pp. 235–242., doi: 10.1016/j.jpowsour.2007.02.065.
Q. What Is Zeolite?
A. Zeolite can be known as mesoporous, a crystalline framework that is manufactured from aluminium oxygen tetrahedra and silicon. However, it refers to a particular type called hierarchical zeolites, which are synthesized to include mesopores (2-50nm) in addition to the characteristic micropores(<2 nm).
FAQ
Q. What Is Zeolite Used for?
A. The zeolite is helpful in gas separation, water purification, fuel cells, and catalysis. However, molecular sieves trap or let through molecules by size.
Q. What Does Zeolite Do for the Body?
A. Zeolite is considered to help the body by trapping harmful chemicals, heavy metals, and toxins in the digestive tract and ensuring safe excretion. Additionally, it becomes beneficial to support gut health and balance pH levels. Still, the scientific evidence on these benefits is limited.
Q. Does Zeolite Work?
A. Yes, zeolites work as drying agents in water, air purifiers, and drying agents. Despite this, zeolite is marketed as a dietary supplement to treat diarrhea, autism, cancer, and balance pH levels; however, there is no scientific evidence to support its effectiveness in any of these conditions.
Q. What Is Zeolite Made of?
A. Zeolite is composed of a 3D network of Silicon dioxide and ammonium sulfate. Tiny positively charged particles balance these structures, that known as cations. It includes sodium and calcium, which sit inside the pores to maintain the electrical balance.
Q. Is Zeolite Safe for Kids?
A. Zeolite safety for children depends on the type and its formulation. Some forms of zeolite pose a risk, but mineral supplements that include refined zeolite, specifically clinoptilolite, are used to support children's digestion. This form doesn't resemble zeolite as a dust.
Q. What Is Zeolite Good for?
A. The main benefit of zeolite is the ability to act as a powerful mineral that can trap and remove toxins. It can even remove the heavy metals and other impurities from the body through adsorption.
Q. How Does Zeolite Work?
A. It works through its porus structure and negative framework, and it adsorbs and exchanges ions, and selectively filters molecules depending on the charge or size.