High Surface Area Graphene Oxide
Graphene oxide with high surface area.
Product Detail
Check out this similar product: High Density Graphene Oxide
NOTE: We are currently updating our SKU system, please contact us if you have any questions.
CAS No.: 7782-42-5
1. Preparation Method: Modified HUMMER’S method
2. Characterizations
|
Type: |
*Type A |
Type B |
|
Appearance: |
Brownish Yellow Powder |
Brownish Grey Powder |
|
Purity: |
~ 99% |
~ 99% |
|
Lateral size: |
1-5 μm |
0.2-10 μm |
|
Thickness: |
0.8-1.2 nm |
~1 nm |
|
Monolayer Rate: |
~ 99% |
>90% |
|
Carbon Content: |
~51.26 wt.% |
~46 wt.% |
|
Oxygen Content: |
~40.78 wt.% |
~50 wt.% |
|
Sulfur Content: |
N/A |
<1.5 wt.% |
|
Density: |
~270g/L |
~290g/L |
| BET: |
>100 m2/g |
~400 m2/g |
* Type A: NEW SKU#: GNOP1101 / OLD SKU#: GnOP1LHS-0.5g

Photos of High Surface Area Graphene Oxide
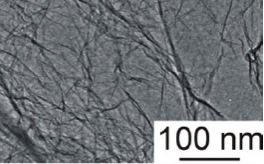
*TEM of High Surface Area Graphene Oxide (Type A)
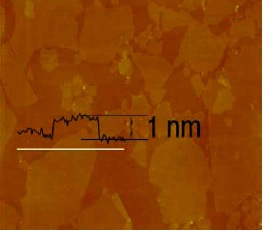
*AFM of High Surface Area Graphene Oxide (Type A)
*The TEM and AFM analysis were completed through dispersing ACS-Material Graphene Oxide into water or ethanol with the help of ultrasound

Typical SEM Image of ACS Material High Surface Area Graphene Oxide (Type B)
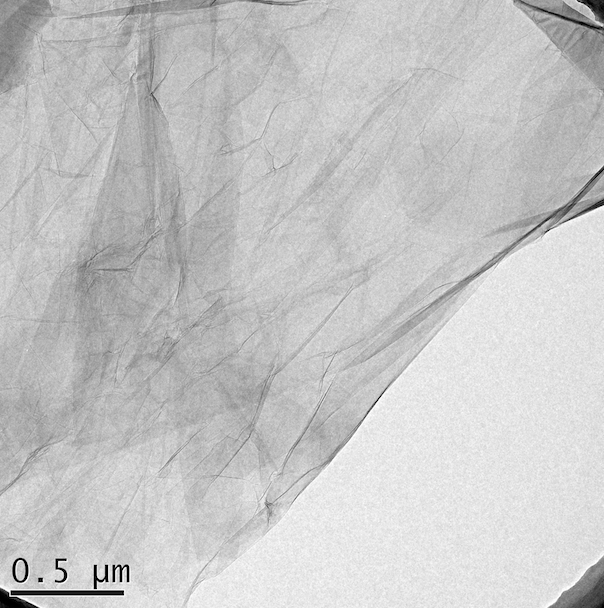
Typical TEM Image of ACS Material High Surface Area Graphene Oxide (Type B)
Application Fields
Supercapacitors; Catalyst; Solar energy; Graphene semiconductor chips; Conductive graphene film; Graphene computer memory; Biomaterials; Transparent conductive coatings.
Graphene oxide will be supplied as a loose powder‚ and it has good solubility in water‚ ethanol‚ DMF et al. The dispersion concentration of graphene oxide with high surface area in water will be larger than 5 mg/ml. Graphene oxide can be easily dispersed in polar solvents with the help of ultrasound. If you have any questions‚ please contact with us‚ and we will try our best to provide the solutions for you.
Attentions
This graphene oxide with high surface area is easier to be oxidized than the normal graphene oxide‚ and it should be kept out of the sun.
*The research demonstrates that a common contaminant (some potassium salt residues) in graphite oxide renders the material highly flammable. The properties of graphene oxide are similar with graphite oxide. ACS Material-Graphene oxide has been purified for many times‚ and the potassium salt is removed completely. Therefore the consumers can use ACS Material-Graphene oxide without any hesitation.
*Kim F‚ Luo J‚ Cruz-Silva R et al. Self-propagating domino-like reactions in oxidized graphite‚ Advanced Functional Materials‚ 2010‚ 20 (17): 2867-2873.
Disclaimer: ACS Material LLC believes that the information on our website is accurate and represents the best and most current information available to us. ACS Material makes no representations or warranties either express or implied, regarding the suitability of the material for any purpose or the accuracy of the information listed here. Accordingly, ACS Material will not be responsible for damages resulting from use of or reliance upon this information.
FAQ
How do I disperse single layer Graphene Oxide into a suitable carrier?
For dispersion in water
1) Use distilled or deionized water
2) Add graphene powder
3) Use sonication power of 200W for about 2 hours
For dispersion in ethanol
1) Use pure ethanol
2) Add graphene powder
3) Use sonication power of 200W for about 4 hours
Is Graphene Oxide fully oxidized? What is the C:O ratio?
Graphene Oxide (GO) is produced by the classic modified Hummer’s method‚ which yields an oxidization level of ≥95%. Regarding the C:O ratio‚ there is some variance inherent in the manufacturing process but the figures below give you an approximation of what to expect:
Sample N (wt %) C (wt %) O (wt %) C/O at. ratio
Graphene Oxide 0 51.26 40.78 1.67
NOTE: This graphene oxide with high surface area is easier to be oxidized than the normal graphene oxide‚ and it should be kept out of the sun.
Research Citations of ACS Material Products
- Mukherjee, Rahul, et al. “Photothermally Reduced Graphene as High-Power Anodes for Lithium-Ion Batteries.” ACS Nano, vol. 6, no. 9, 2012, pp. 7867–7878., doi:10.1021/nn303145j.
- Chow, Philippe K., et al. “Mechanical Property Enhancement of Layered Reduced Graphene Oxide Papers by Non-Covalent Modification with Terephthalic Acid.” Particle & Particle Systems Characterization, vol. 31, no. 3, 2013, pp. 337–341., doi:10.1002/ppsc.201300206.
- Feng, Xuefei, et al. “Understanding the degradation mechanism of rechargeable lithium/Sulfur cells: a comprehensive study of the sulfur–graphene oxide cathode after discharge–charge cycling.” Phys. Chem., vol. 16, no. 32, 2014, pp. 16931–16940., doi:10.1039/c4cp01341g.
- Cui, Shumao, et al. “Ultrasensitive Chemical Sensing through Facile Tuning Defects and Functional Groups in Reduced Graphene Oxide.” Analytical Chemistry, vol. 86, no. 15, Nov. 2014, pp. 7516–7522., doi:10.1021/ac501274z.
- Mukherjee, Rahul, et al. “Defect-Induced plating of lithium metal within porous graphene networks.” Nature Communications, vol. 5, 2014, doi:10.1038/ncomms4710.
- Lammel, Tobias, et al. “Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2.” Particle and Fibre Toxicology, vol. 10, no. 1, 2013, p. 27., doi:10.1186/1743-8977-10-27.
- Cheng, Jianli, et al. “Self-Assembled V2O5 nanosheets/Reduced graphene oxide hierarchical nanocomposite as a high-Performance cathode material for lithium ion batteries.” Journal of Materials Chemistry A, vol. 1, no. 36, 2013, p. 10814., doi:10.1039/c3ta12066j.
- Li, Shanghao, et al. “Interaction between Graphene Oxide and Pluronic F127 at the Air–Water Interface.” Langmuir, vol. 29, no. 19, Feb. 2013, pp. 5742–5748., doi:10.1021/la401056t.
- Othman, Abdelhameed M., et al. “Enhancing selectivity in spectrofluorimetric determination of tryptophan by using graphene oxide nanosheets.” Analytica Chimica Acta, vol. 787, 2013, pp. 226–232., doi:10.1016/j.aca.2013.05.036.
- Zhang, Xu, et al. “Effects of Polyethylene Glycol on DNA Adsorption and Hybridization on Gold Nanoparticles and Graphene Oxide.” Langmuir, vol. 28, no. 40, 2012, pp. 14330–14337., doi:10.1021/la302799s.
- Li, Shanghao, et al. “Graphene Oxide as a Quencher for Fluorescent Assay of Amino Acids, Peptides, and Proteins.” ACS Applied Materials & Interfaces, vol. 4, no. 12, Mar. 2012, pp. 7069–7075., doi:10.1021/am302704a.
- Ip, Alexander C.-F., et al. “Oxidation Level-Dependent Zwitterionic Liposome Adsorption and Rupture by Graphene-Based Materials and Light-Induced Content Release.” Small, vol. 9, no. 7, 2012, pp. 1030–1035., doi:10.1002/smll.201202710.
- Chang, Jingbo, et al. “Ultrasonic-Assisted self-Assembly of monolayer graphene oxide for rapid detection of Escherichia coli bacteria.” Nanoscale, vol. 5, no. 9, 2013, p. 3620., doi:10.1039/c3nr00141e.
- Liu, Lin, et al. “Deposition and transport of graphene oxide in saturated and unsaturated porous media.” Chemical Engineering Journal, vol. 229, 1 Aug. 2013, pp. 444–449., doi:10.1016/j.cej.2013.06.030.
- Li, Shanghao, et al. “Head Groups of Lipids Govern the Interaction and Orientation between Graphene Oxide and Lipids.” The Journal of Physical Chemistry C, vol. 117, no. 31, 2013, pp. 16150–16158., doi:10.1021/jp405991q.
- Tran, H. V., et al. “Antibodies Directed to RNA/DNA Hybrids: An Electrochemical Immunosensor for MicroRNAs Detection using Graphene-Composite Electrodes.” Analytical Chemistry, vol. 85, no. 17, 2013, pp. 8469–8474., doi:10.1021/ac402154z.
- Liu, Biwu, et al. “Mechanisms of DNA Sensing on Graphene Oxide.” Analytical Chemistry, vol. 85, no. 16, June 2013, pp. 7987–7993., doi:10.1021/ac401845p.
- Luan, Xinning, et al. “Electrophoretic deposition of reduced graphene oxide nanosheets on TiO2 nanotube arrays for dye-Sensitized solar cells.” Electrochimica Acta, vol. 111, 2013, pp. 216–222., doi:10.1016/j.electacta.2013.08.016.
- Zhang, Ming, et al. “Synthesis of a multifunctional graphene–carbon nanotube aerogel and its strong adsorption of lead from aqueous solution.” RSC Advances, vol. 3, no. 43, 2013, p. 21099., doi:10.1039/c3ra44340j.
- Wu, Lei, et al. “Aggregation Kinetics of Graphene Oxides in Aqueous Solutions: Experiments, Mechanisms, and Modeling.” Langmuir, vol. 29, no. 49, Mar. 2013, pp. 15174–15181., doi:10.1021/la404134x.
- Wang, Feng, and Juewen Liu. “Nanodiamond decorated liposomes as highly biocompatible delivery vehicles and a comparison with carbon nanotubes and graphene oxide.” Nanoscale, vol. 5, no. 24, 2013, p. 12375., doi:10.1039/c3nr04143c.
- Zhang, Ming, et al. “Simple approach for large-Scale production of reduced graphene oxide films.” Chemical Engineering Journal, vol. 243, 2014, pp. 340–346., doi:10.1016/j.cej.2014.01.019.
- Frechette, M. F., et al. “Preparation and dielectric responses of solid epoxy composites containing a mixture of epoxy powder ball-Milled with GO.” 2013 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, 2013, doi:10.1109/ceidp.2013.6748335.
- Chaturvedi, P. et al. “A nanoceria–platinum–graphene nanocomposite for electrochemical biosensing.” Biosensors and Bioelectronics, vol. 58, 15 Aug. 2014, doi:10.1016/j.bios.2014.02.021.
- Lammel, Tobias, and José M. Navas. “Graphene nanoplatelets spontaneously translocate into the cytosol and physically interact with cellular organelles in the fish cell line PLHC-1.” Aquatic Toxicology, vol. 150, 2014, pp. 55–65., doi:10.1016/j.aquatox.2014.02.016.
- Souza, Camille De, et al. “Electrocatalytic miRNA Detection Using Cobalt Porphyrin-Modified Reduced Graphene Oxide.” Sensors, vol. 14, no. 6, June 2014, pp. 9984–9994., doi:10.3390/s140609984.
- Lammel, Tobias, et al. “Potentiating effect of graphene nanomaterials on aromatic environmental pollutant-Induced cytochrome P450 1A expression in the topminnow fish hepatoma cell line PLHC-1.” Environmental Toxicology, vol. 30, no. 10, May 2014, pp. 1192–1204., doi:10.1002/tox.21991.
- Tran, H.V., et al. “An electrochemical ELISA-like immunosensor for miRNAs detection based on screen-Printed gold electrodes modified with reduced graphene oxide and carbon nanotubes.” Biosensors and Bioelectronics, vol. 62, 2014, pp. 25–30., doi:10.1016/j.bios.2014.06.014.
- Wang, Feng, and Juewen Liu. “Platinated DNA oligonucleotides: new probes forming ultrastable conjugates with graphene oxide.” Nanoscale, vol. 6, no. 12, 2014, p. 7079., doi:10.1039/c4nr00867g.
- Chen, Hao, et al. “Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide.” Journal of Hazardous Materials, vol. 282, 2015, pp. 201–207., doi:10.1016/j.jhazmat.2014.03.063.
- Zhang, Ming, et al. “Slow-Release fertilizer encapsulated by graphene oxide films.” Chemical Engineering Journal, vol. 255, 2014, pp. 107–113., doi:10.1016/j.cej.2014.06.023.
- He, Guang, et al. “Stable Cycling of a Scalable Graphene-Encapsulated Nanocomposite for Lithium–Sulfur Batteries.” ACS Applied Materials & Interfaces, vol. 6, no. 14, May 2014, pp. 10917–10923., doi:10.1021/am500632b.
- Li, Shanghao, et al. “Strong and Selective Adsorption of Lysozyme on Graphene Oxide.” ACS Applied Materials & Interfaces, vol. 6, no. 8, Aug. 2014, pp. 5704–5712., doi:10.1021/am500254e.
- Vanegas, D. C., et al. “Xanthine oxidase biosensor for monitoring meat spoilage.” Smart Biomedical and Physiological Sensor Technology XI, 2014, doi:10.1117/12.2050489.
- Nguyen, Le Huy, et al. “Functionalization of reduced graphene oxide by electroactive polymer for biosensing applications.” Advances in Natural Sciences: Nanoscience and Nanotechnology, vol. 5, no. 3, Sept. 2014, p. 035005., doi:10.1088/2043-6262/5/3/035005.
- Li, Shanghao. “Analysis of the Interactions between Graphene Oxide and Biomolecules and Protein Fibrillation Using Surface Chemistry and Spectroscopy.” University of Miami Scholarly Repository.
- Ding, Zhuhong, et al. “Filtration and transport of heavy metals in graphene oxide enabled sand columns.” Chemical Engineering Journal, vol. 257, 2014, pp. 248–252., doi:10.1016/j.cej.2014.07.034.
- Sims, Christopher M., et al. “CO tolerance of Pt and PtSn intermetallic electrocatalysts on synthetically modified reduced graphene oxide supports.” Dalton Transactions, vol. 44, no. 3, 2015, pp. 977–987., doi:10.1039/c4dt02544j.
- Sun, Yuanyuan, et al. “Transport, retention, and size perturbation of graphene oxide in saturated porous media: Effects of input concentration and grain size.” Water Research, vol. 68, 2015, pp. 24–33., doi:10.1016/j.watres.2014.09.025.
- Feng, Xuefei, et al. “Understanding the degradation mechanism of rechargeable lithium/Sulfur cells: a comprehensive study of the sulfur–graphene oxide cathode after discharge–charge cycling.” Phys. Chem., vol. 16, no. 32, 2014, pp. 16931–16940., doi:10.1039/c4cp01341g.
- Luan, Xinning, et al. “Enhanced photocatalytic activity of graphene oxide/Titania nanosheets composites for methylene blue degradation.” Materials Science in Semiconductor Processing, vol. 30, 2015, pp. 592–598., doi:10.1016/j.mssp.2014.10.032.
- Burrs, S. L. “A comparative study of graphene–hydrogel hybrid bionanocomposites for biosensing.” Analyst, no. 5, 24 Dec. 2014, doi:10.1039/C4AN01788A.
- Tangorra, Rocco Roberto, et al. “Photoactive film by covalent immobilization of a bacterial photosynthetic protein on reduced graphene oxide surface.” MRS Proceedings, vol. 1717, 2015, doi:10.1557/opl.2015.18.
- Huang, Taizhong, et al. “A high-Performance catalyst support for methanol oxidation with graphene and vanadium carbonitride.” Nanoscale, vol. 7, no. 4, 2015, pp. 1301–1307., doi:10.1039/c4nr05244g.
- Chen, Hang, and Tobin Filleter. “Effect of structure on the tribology of ultrathin graphene and graphene oxide films.” Nanotechnology, vol. 26, no. 13.
- Huynh, Vien T., et al. “Polymer coating of graphene oxide via reversible addition-Fragmentation chain transfer mediated emulsion polymerization.” Journal of Polymer Science Part A: Polymer Chemistry, vol. 53, no. 12, 2015, pp. 1413–1421., doi:10.1002/pola.27596.
- Cui, Shumao, et al. “Stabilizing MoS2Nanosheets through SnO2Nanocrystal Decoration for High-Performance Gas Sensing in Air.” Small, vol. 11, no. 19, 2015, pp. 2305–2313., doi:10.1002/smll.201402923.
- Gao, Xianfeng, et al. “A Multilayered Silicon-Reduced Graphene Oxide Electrode for High Performance Lithium-Ion Batteries.” ACS Applied Materials & Interfaces, vol. 7, no. 15, 2015, pp. 7855–7862., doi:10.1021/acsami.5b01230.
- Huang, Po-Jung Jimmy, et al. “Inhibiting the VIM-2 Metallo-β-Lactamase by Graphene Oxide and Carbon Nanotubes.” ACS Applied Materials & Interfaces, vol. 7, no. 18, 2015, pp. 9898–9903., doi:10.1021/acsami.5b01954.
- Zhao, Yingcan, and Chad T. Jafvert. “Environmental photochemistry of single layered graphene oxide in water.” Environmental Science: Nano, vol. 2, no. 2, 2015, pp. 136–142., doi:10.1039/c4en00209a.
- Liang, Xiao, et al. “A highly efficient polysulfide mediator for lithium–sulfur batteries.” Nature Communications, vol. 6, June 2015, p. 5682., doi:10.1038/ncomms6682.
- Vanegas, D. C., et al. “Rapid detection of listeria spp. using an internalin A aptasensor based on carbon-Metal nanohybrid structures.” Smart Biomedical and Physiological Sensor Technology XII, 2015, doi:10.1117/12.2177441.
- Vanegas, Diana C., et al. “A self-Referencing biosensor for real-Time monitoring of physiological ATP transport in plant systems.” Biosensors and Bioelectronics, vol. 74, 2015, pp. 37–44., doi:10.1016/j.bios.2015.05.027.
- Huang, Po-Jung Jimmy, et al. “Hg2 detection using a phosphorothioate RNA probe adsorbed on graphene oxide and a comparison with thymine-Rich DNA.” The Analyst, vol. 141, no. 12, 2016, pp. 3788–3793., doi:10.1039/c5an02031j.
- El-Amin, Ayman A., and Abdelhameed M. Othman. “Novel GO-LaSmO2 Nanocomposite as an Effective Electrode Material for Hydrogen Fuel Cells.” Jom, vol. 68, no. 4, Aug. 2015, pp. 1209–1215., doi:10.1007/s11837-015-1741-9.
- Song, Min-Kyu, et al. “Effects of cell construction parameters on the performance of lithium/Sulfur cells.” AIChE Journal, vol. 61, no. 9, July 2015, pp. 2749–2756., doi:10.1002/aic.14947.
- Dong, Shunan, et al. “Graphene oxide as filter media to remove levofloxacin and lead from aqueous solution.” Chemosphere, vol. 150, 2016, pp. 759–764., doi:10.1016/j.chemosphere.2015.11.075.
- Chang, Jingbo, et al. “Real-Time detection of mercury ions in water using a reduced graphene oxide/DNA field-Effect transistor with assistance of a passivation layer.” Sensing and Bio-Sensing Research, vol. 5, 2015, pp. 97–104., doi:10.1016/j.sbsr.2015.07.009.
- Xu, Wangwang, et al. “Hierarchical Graphene-Encapsulated Hollow SnO2@SnS2 Nanostructures with Enhanced Lithium Storage Capability.” ACS Applied Materials & Interfaces, vol. 7, no. 40, 2015, pp. 22533–22541., doi:10.1021/acsami.5b06765.
- Ye, Yifan. “X-Ray Absorption Spectroscopy Characterization of a Li/S Cell.” Nanomaterials, 2016, doi:10.3390/nano6010014.
- Nguyen, Trang H. D., et al. “Toxicity of Graphene Oxide on Intestinal Bacteria and Caco-2 Cells.” Journal of Food Protection, vol. 78, no. 5, 2015, pp. 996–1002., doi:10.4315/0362-028x.jfp-14-463.
- Huang, Po-Jung Jimmy, et al. “Liposome/Graphene Oxide Interaction Studied by Isothermal Titration Calorimetry.” Langmuir, vol. 32, no. 10, Apr. 2016, pp. 2458–2463., doi:10.1021/acs.langmuir.6b00006.
- Liu, Biwu, et al. “Graphene oxide surface blocking agents can increase the DNA biosensor sensitivity.” Biotechnology Journal, vol. 11, no. 6, Nov. 2016, pp. 780–787., doi:10.1002/biot.201500540.
- Liu, Taoze, et al. “Biochar-Supported carbon nanotube and graphene oxide nanocomposites for Pb(Ii) and Cd(Ii) removal.” RSC Advances, vol. 6, no. 29, 2016, pp. 24314–24319., doi:10.1039/c6ra01895e.
- Nguyen, T. Dung, et al. “One-Step Electrosynthesis of Poly(1,5-Diaminonaphthalene)/Graphene Nanocomposite as Platform for Lead Detection in Water.” Electroanalysis, vol. 28, no. 8, Nov. 2016, pp. 1907–1913., doi:10.1002/elan.201501075.
- Tegou, E., et al. “Low-Temperature thermal reduction of graphene oxide films in ambient atmosphere: Infra-Red spectroscopic studies and gas sensing applications.” Microelectronic Engineering, vol. 159, 2016, pp. 146–150., doi:10.1016/j.mee.2016.03.030.
- Xu, Wangwang, et al. “Direct growth of an economic green energy storage material: a monocrystalline jarosite-KFe3(SO4)2(OH)6-Nanoplates@RGO hybrid as a superior lithium-Ion battery cathode.” Journal of Materials Chemistry A, vol. 4, no. 10, 2016, pp. 3735–3742., doi:10.1039/c5ta10622b.
- Pandey, Gaind P. “Mesoporous Hybrids of Reduced Graphene Oxide and Vanadium Pentoxide for Enhanced Performance in Lithium-Ion Batteries and Electrochemical Capacitors.” Applied Materials & Interfaces, 24 Mar. 2016, pp. 9200–9210., doi:10.1021/acsami.6b02372.
- Nieto, Andy, et al. “Graphene reinforced metal and ceramic matrix composites: a review.” International Materials Reviews, vol. 62, no. 5, 2016, pp. 241–302., doi:10.1080/09506608.2016.1219481.
- Wang, Mei, et al. “Concurrent aggregation and transport of graphene oxide in saturated porous media: Roles of temperature, cation type, and electrolyte concentration.” Environmental Pollution, vol. 235, 2018, pp. 350–357., doi:10.1016/j.envpol.2017.12.063.
- Berning, T.; Bessarabov, D. GOMEA: A Conceptual Design of a Membrane Electrode Assembly for a Proton Exchange Membrane Electrolyzer. Membranes 2023, 13, 614. https://doi.org/10.3390/membranes13070614

