-
Black Titanium Dioxide
Jan 16, 2019 | ACS MATERIAL LLCTitanium Dioxide (TiO2) nanomaterials have been used primarily as photocatalysts over past few decades, however, its wide band gap largely limited TiO2 activity to the UV region of the solar spectrum. The discovery of ‘‘black’’ titanium dioxide nanoparticles with visible infrared absorption opened up all new possibilities in this sector. Since the discovery of black titanium dioxide, world-wide research initiatives have been launched for the purpose of narrowing the band gap and overcoming that issue. Success in this endeavor could positively affect a wide array of applications in a diverse set of solar energy systems.
Introduction
In 2011, black TiO2 was (TiO2-x) as reported by Xiaobo Chen’s research group studying hydrogenation.1 At the time, we had come to know that conventional titanium dioxide (TiO2) nanomaterials had a wide band gap of 3.2 eV (387 nm) and 3.0 eV (413 nm), absorbing only UV light or less than 5% of full solar energy. Black TiO2-x proved superior by exhibiting the optical absorption onset extended into the infrared region (~1,150 nm). It turned out that reduction by the hydrogenation method dramatically changed the structural, chemical, electronic and optical properties of TiO2 nanoparticles for the better. After hydrogenation, crystalline/disordered and core/shell nanoparticles were obtained with long-wavelength absorption, low structural and chemical defects, and enhanced electrical conductivity. These enhanced properties have attracted great interest in black TiO2-x nanomaterials ever since.
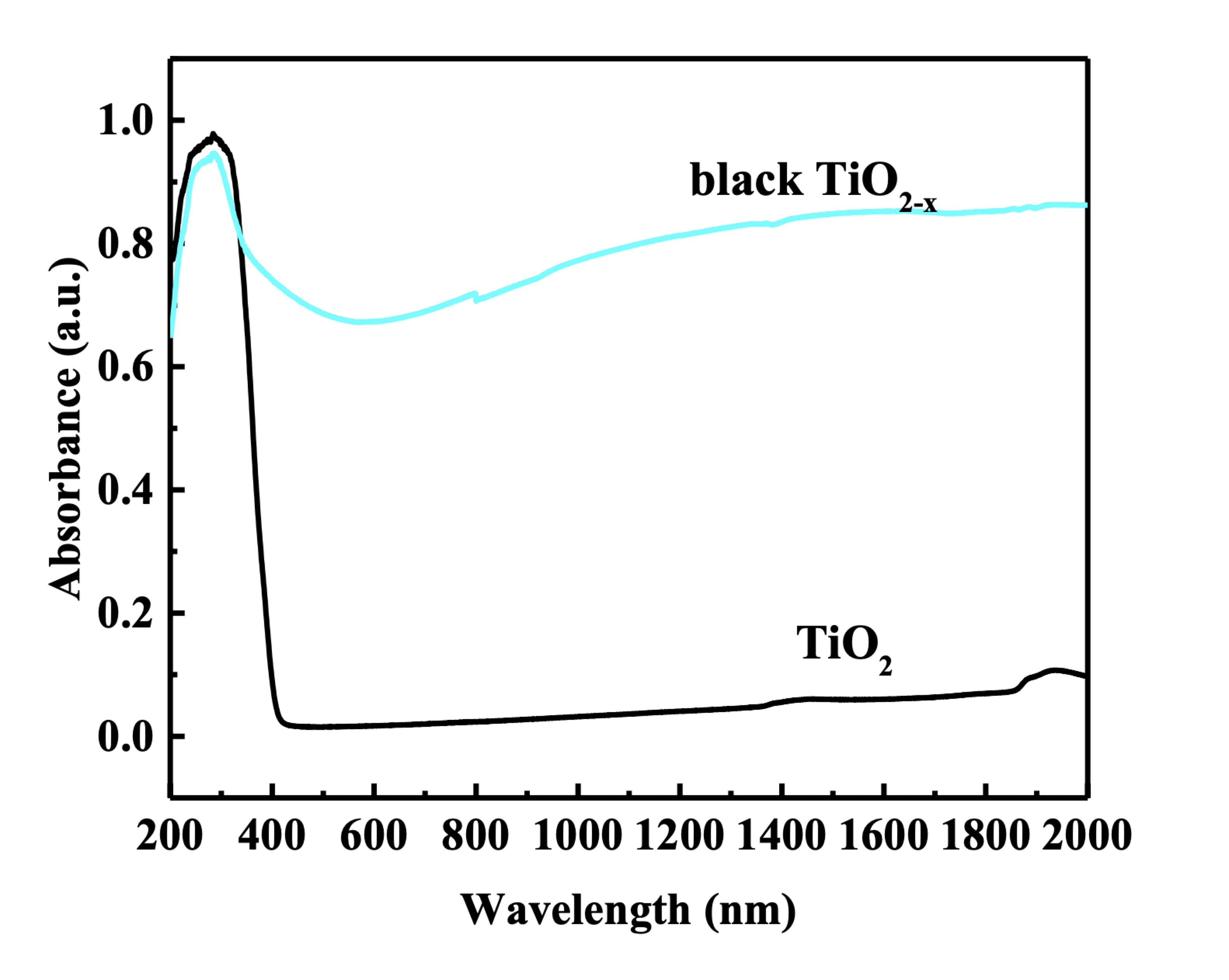
Figure 1. UV-Vis-NIR absorption spectra of TiO2 and black TiO2-x
Synthesis
Hydrogenation is a simple and straightforward method that has been used to introduce Ti3+ and other reduction states. This method involves treatment under an environment containing hydrogen or hydrogen plasma for an indicated amount of time at specific temperatures. The interaction between hydrogen and TiO2 has been thoroughly investigated by means of this method. Besides the hydrogenation method, however, other chemical reductionmethods including aluminum reduction, magnesium reduction, NaBH4 reduction and NaH reduction have also been exploited to study the preparation of black TiO2-x in greater detail 2-4.

Figure 2. Images of TiO2 and black TiO2-x powders.
Generally, black TiO2-x nanoparticles are obtained by hydrogenation at high pressure for an extended period of time (20.0 bar H2, 200 °C, 5 days; 1bar=0.1 MPa). Black TiO2-x, as available on our ACS Material online store, is obtained by reduction with NaBH4.4-5 The starting TiO2 is prepared by reacting 5 mL of 50 wt% titanium (IV) bis(ammonium lactate) dihydroxide and 60 mL 0.08 g/L glucose at 170 °C for 8 hours, followed by annealing at 500 °C for 3 hours. 12g of NaBH4 and 0.5g TiO2 are then dispersed in 60 mL of mixed TiO2 nanoparticles (4.0 g) and NaBH4 (1.5 g) powders reacted at 400-900 °C for 60-180 minutes. Adjusting the reaction temperature and time leads to a series of TiO2 compounds of varying colors. A crystalline core/amorphous shell structure can be observed with significant visible-light absorption. Images of TiO2 and reduced black TiO2-x powders are shown in Figure 2 and corresponding XRD patterns are shown in Figure 3 below. The XRD pattern for TiO2 shows sharp peaks, which indicates good crystallinity. However, after reduction with NaBH4, the crystallinity of black TiO2-x decreases significantly.
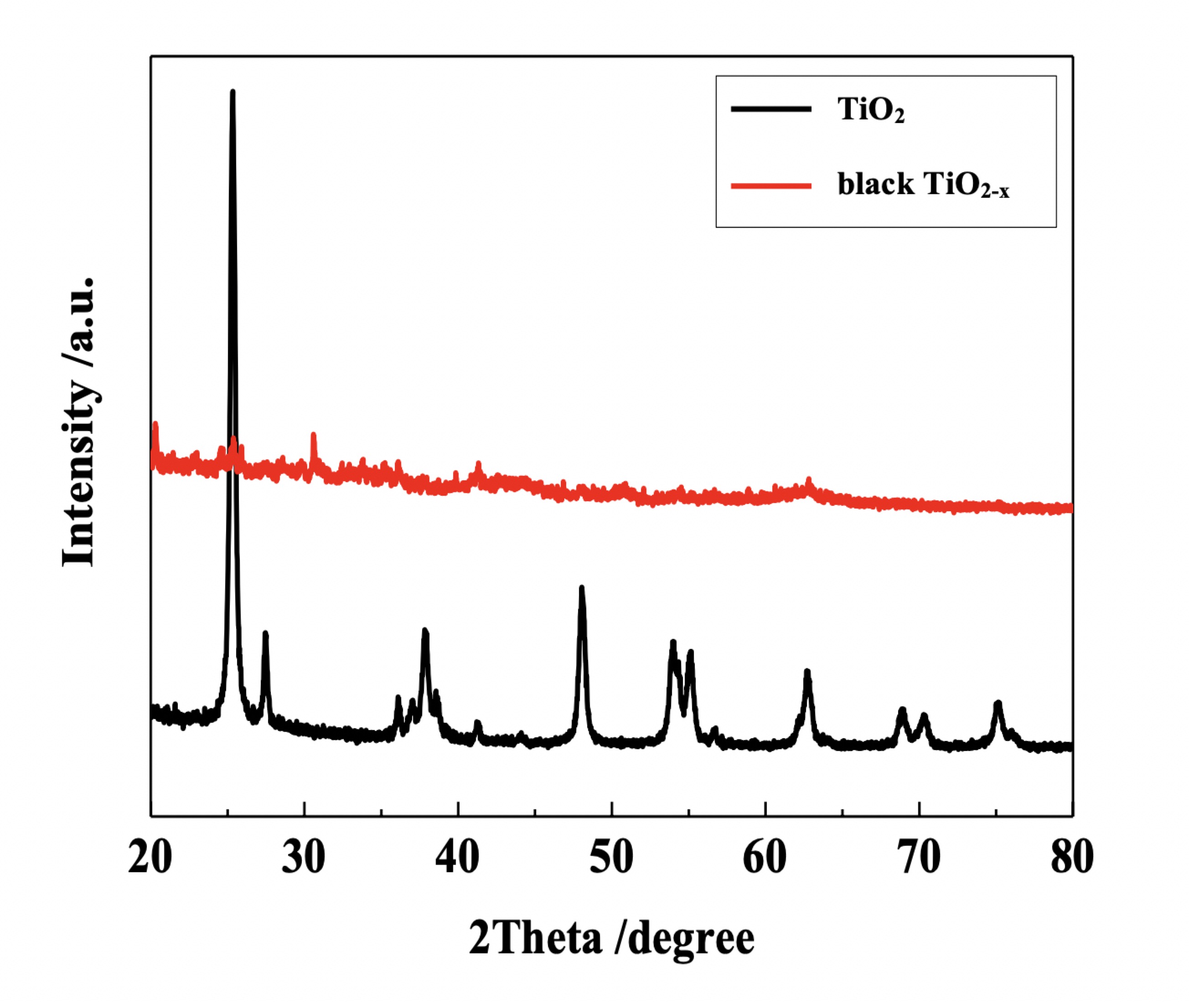
Figure 3. XRD patterns of TiO2 and black TiO2-X
Applications
Higher photocatalytic activities in generating H2 from water-methanol solution anddecomposing methylene blue and phenol were also reported of the hydrogenated black TiO2-x nanoparticles. For example, excellent efficiencies were reported forthe nanoporous black TiO2-x in the photocatalytic degradation of phenol, rhodamine B, andmethylene blue.6 Excellent photocatalytic activity in the degradation of organic molecules inwater was obtained with black TiO2-x prepared by hydrogen plasma assisted chemical vapor deposition, asreported by Teng F.7
In 2015 Ren W. demonstrated that hydrogenated black TiO2-x nanoparticles coated with polyethylene glycol (PEG) showed good potential in cancer diagnosis and therapy.8 A photothermal conversion efficiency of 40.8% was obtained with a low toxicity and a high anti-cancer effect invitro and in vivo. A superior photothermal therapy effect was found inan in vivo mouse model using deepblue Ti8O15 nanoparticles with >98% absorption of near infrared light.9 Cancer cells were effectively eliminated after 808nm laser irradiation for 5 minutes and low biotoxicity was observed.
Furthermore, an excellent rate performance of lithium storage was observed in black TiO2-x nanocrystals due to the well-balanced Li+/e- diffusion.10 The high capacity polyaniline/black TiO2-x nanotube electrode possess long-term cycling stability, which can be due to the oxygen vacancies (Ti3+sites) created during the hydrogenation process.10
Black TiO2-x is simultaneously being studied for its potential use in applications such as lithium-ion batteries, supercapacitors, fuel cells and microwave absorption.10
Conclusion
Black TiO2-x nanomaterials have undergone scientific research since its discovery in 2011 and has demonstrated great potential for many applications now and into the future. Over the past decade, a series of synthetic methods for producing black TiO2-x have been developed such as the hydrogen thermal treatment, hydrogen plasma, chemical reduction, electrochemical reduction etc.. These enriched methods have provided flexibility in tuning size, morphology, properties and performance of black TiO2-x. Additional efforts including experimental and theoretical studies are needed to advance this material further towards more real-world applications.
ACS Material Products:
References
1. Chen X., Liu L., Peter Y. Y., Mao S. S., Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals.Science 2011, 331, 746-750.
2. Wallace W. E., Zhong Q., Genzer J., On the use of ion scattering to examine the role of hydrogen in the reduction of TiO2. J. Mater. Res. 2011, 8, 1629-1634.
3. Becker J. H., Hosler W. R., Multiple-band conduction in n-type rutile (TiO2). Phys. Rev. A 1965, 137: 1872-1877.
4. Liu X. H., Hou B. F., Wang G., Cui Z. Q., Zhu X., and Wang X. B., Black titania/graphene oxide nanocomposite films with excellent photothermal property for solar steam generation, J. Mater. Res., 2018, 33, 674-684
5. Tan H., Zhao Z., Niu M., A facile and versatile method for preparation of colored TiO2with enhanced solar-driven photocatalytic activity. Nanoscale, 2014, 6: 10216-10223.
6. An H. R., Park S. Y., Kim H., Advanced nanoporous TiO2photocatalysts by hydrogen plasma for efficient solar-light photocatalytic application. Sci. Rep. 2016, 6:29683.
7. Teng F., Li M., Gao C., Preparation of black TiO2 by hydrogen plasma assisted chemical vapor deposition and its photocatalytic activity. Appl. Catal. B Environ. 2014, 148-149: 339-343.
8. Ren W. Yan Y., Zeng L., A near infrared light triggered hydrogenated black TiO2for cancer photothermal therapy. Adv. Healthcare. Mater. 2015, 4: 1526-1536.
9. Ou G., Li Z., Li D., Photothermal therapy by using titanium oxide nanoparticles. Nano Res., 2016, 9: 1236-1243.
10. Liu Y., Tian L. H., Tan X. Y., Li X., Chen X. B., Synthesis, properties, and applications of black titanium dioxide nanomaterials, Science Bulletin , 2017, 6 : 431-441.